aluminum oxide decomposition balanced equation
 3) Given a 10.1M stock solution, how many mL must be added to water to produce 200 mL of 5M solution? 3MnO2 +4Al--> 3Mn+2Al2O3? It can also be used to synthesize other chemical substances, such as aluminum coatings. The balanced chemical equation for this reaction is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. Label Each Compound With a Variable Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. What is the ratio of the numbers of molecules in these two samples? The reaction is exothermic, which means that it releases heat. Since all the moles of C and H in CO2 and H2O, respectively have to have came from the 1 gram sample of unknown, start by calculating how many moles of each element were present in the unknown sample. There are many different strategies that people use in order to balance chemical equations. Since 3.9984 is very close to four, it is possible to safely round up and assume that there was a slight error in the experimentally determined molecular mass. If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? 0.0333mol CO2 (2mol O/1mol CO2) = 0.0666 mol O, 0.599g H2O (1mol H2O/18.01528 g H2O)(1mol O/1mol H2O) = 0.0332 mol O. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#? Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) . The Activity Series of Metals | Predicting Products of Chemical Reactions. The molecular formula for aluminum bromide shows that this compound is made from one atom of aluminum and three atoms of bromine. Try refreshing the page, or contact customer support. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.
3) Given a 10.1M stock solution, how many mL must be added to water to produce 200 mL of 5M solution? 3MnO2 +4Al--> 3Mn+2Al2O3? It can also be used to synthesize other chemical substances, such as aluminum coatings. The balanced chemical equation for this reaction is: 2 Al(s) + 3 Br2(l) ==> 2 AlBr3(s). Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. Label Each Compound With a Variable Label each compound (reactant or product) in the equation with a variable to represent the unknown coefficients. What is the ratio of the numbers of molecules in these two samples? The reaction is exothermic, which means that it releases heat. Since all the moles of C and H in CO2 and H2O, respectively have to have came from the 1 gram sample of unknown, start by calculating how many moles of each element were present in the unknown sample. There are many different strategies that people use in order to balance chemical equations. Since 3.9984 is very close to four, it is possible to safely round up and assume that there was a slight error in the experimentally determined molecular mass. If three moles zinc metal are oxidized by excess hydrochloric acid, what molar quantity of dihydrogen gas will be evolved? 0.0333mol CO2 (2mol O/1mol CO2) = 0.0666 mol O, 0.599g H2O (1mol H2O/18.01528 g H2O)(1mol O/1mol H2O) = 0.0332 mol O. How many moles of #HNO_3# will be produced when .51 mol of #N_2O_5# reacts in the equation #N_2O_5 + H_2O -> 2HNO_3#? Step 1: 200 g \(C_3H_8\) is equal to 4.54 mol \(C_3H_8\) . The Activity Series of Metals | Predicting Products of Chemical Reactions. The molecular formula for aluminum bromide shows that this compound is made from one atom of aluminum and three atoms of bromine. Try refreshing the page, or contact customer support. In Greek, stoikhein means element and metron means measure, so stoichiometry literally translated means the measure of elements. Write a balanced chemical equation for the reactions given below: This page titled 5.3: Balancing Chemical Equations is shared under a CC BY-SA 4.0 license and was authored, remixed, and/or curated by Paul R. Young (ChemistryOnline.com) via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request.  Chemical equation balancer helps you complete the process digitally. How many moles of nitrogen atoms are there in .3400 moles of ammonium nitrate, #NH_4NO_3#? another element Aluminum bromide has a number of important uses.
Chemical equation balancer helps you complete the process digitally. How many moles of nitrogen atoms are there in .3400 moles of ammonium nitrate, #NH_4NO_3#? another element Aluminum bromide has a number of important uses.  Since the numbers are the same, the equation is now balanced. Since the overall charge of a compound must always be equal to zero (neutral), we need 2 aluminum atoms and 3 oxygen atoms in order to balance out the charge and make the compound neutral. 2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide The reaction between barium chloride and sulfuric acid is represented by the equation: BaCl2 + H2SO4 BaSO4 + 2HCl. Polyprotic & Monoprotic Acids Overview & Examples | What is Polyprotic Acid? Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula. Decomposition Reactions. In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. What is the ratio of nitrogen atoms to hydrogen atoms in ammonia? Heating tin (IV) hydroxide gives tin (IV) oxide and water. Subscripts - Part of the chemical formulas of the reactants and products that indicate WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below.
Since the numbers are the same, the equation is now balanced. Since the overall charge of a compound must always be equal to zero (neutral), we need 2 aluminum atoms and 3 oxygen atoms in order to balance out the charge and make the compound neutral. 2Al2O3(l) --> 4Al(l) + 3O2(g), Aluminium + Oxygen = Aluminium Oxide The reaction between barium chloride and sulfuric acid is represented by the equation: BaCl2 + H2SO4 BaSO4 + 2HCl. Polyprotic & Monoprotic Acids Overview & Examples | What is Polyprotic Acid? Multiply the ratio from step 4 by the subscripts of the empirical formula to get the molecular formula. Decomposition Reactions. In this process, aluminium oxide is molten (liquid state) so that ions can move to complete the electricity circuit. What is the ratio of nitrogen atoms to hydrogen atoms in ammonia? Heating tin (IV) hydroxide gives tin (IV) oxide and water. Subscripts - Part of the chemical formulas of the reactants and products that indicate WebThe balanced chemical equation for the decomposition of aluminum oxide resulting in the formation of aluminum and oxygen is shown below. 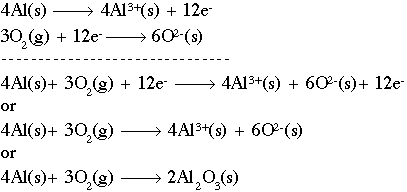 Achieve a common 6 oxygen atoms on either side of the equation. The alloy's density is 3.15 g/L. Why did the Osage Indians live in the great plains? How many party invitations can be sent? This means we may need multiple ions of each to make the compound neutral. Aluminum oxide has the formula Al 2 O 3. Start with the balanced equation for the synthesis of aluminum oxide: 4Al + 3O2 --> 2Al2O3. Making educational experiences better for everyone. One common method for synthesizing aluminum bromide in a chemistry lab is to introduce powdered aluminum into a beaker containing liquid bromine. AB - A + B How are mole ratios used in chemical calculations? If the question had been stated in terms of grams, you would have had to convert grams of HO to moles of HO, then moles of HO to moles of O (as above), and finally moles of O to grams of O. 0.333mol CO2(44.0098g CO2/ 1mol CO2) = 1.466g CO2, 1.466g CO2 + 0.599g H2O - 1.000g unknown organic = 1.065g O2, 1.065g O2(1mol O2/ 31.9988g O2)(2mol O/1mol O2) = 0.0666mol O, (0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol O. Construct a mole ratio for C, H, and O in the unknown and divide by the smallest number. WebThe metal aluminum burns by reacting with oxygen gas to form solid aluminum oxide. How many moles of #"NaOH"# were used to neutralize 0.0220 moles of #"HCl"# if the mole ratio is #"1/1"# ? The general form of a decomposition reaction is: AB A + B. #3(NH_4)_2PtCl_6(s) + Delta rarr 3Pt(s) + 2NH_4Cl(s) + 2N_2(g) +16HCl(g)# Each big cat has 7 small cats. What is the lowest whole number ratio of the elements in a compound called? Each party invitation needs 2 stamps to be sent. experimentally determined mass = 120.056 g/mol, % error = | theoretical - experimental | / theoretical * 100%, % error = | 120.104 g/mol - 120.056 g/mol | / 120.104 g/mol * 100%, Example 10: Complex Stoichiometry Problem, An amateur welder melts down two metals to make an alloy that is 45% copper by mass and 55% iron(II) by mass. What is the mole ratio of #H_2O# to #H_3PO_4# in the following chemical equation? In the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#, what is the ratio of moles of oxygen used to moles of #CO_2# produced? ". If #2.40 xx 10^(-1) # moles of oxygen react, how many moles of carbon dioxide form? 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for calculating limiting reactant. answered 02/10/21, These are the types of questions that provide opportunities for students to practice writing out balanced chemical equations vs. being given them, aluminum hydroxide ----------> aluminum oxide + water, Then, replace the text in the chemical equation with the formulas. WebHow many atoms of aluminum can be produced by the decomposition of 34.6 g of aluminum oxide? It is especially important to pay attention to charge when. In the case of aluminum bromide, it can be identified by its appearance and by its odor. A solution contains 72.0 g of #HCl# and 468 g of #C_6H_6#. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Why are mole ratios central to stoichiometric calculations? Mar 2023 31. marquis grissom baseball academy Facebook; A chemist performs the reaction #6ClO_2 + 3H_2O -> 5HClO_3 + HCl#. WebAluminum Hydrogen Carbonate (aq) B. Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. These methods are generally useful for most simple chemical equations, although mathematical algorithms are often necessary for highly complex reactions. How many moles of #I_2# will form 3.58 g of #NI_3#? Invitation needs 2 stamps to be sent enrolling in a aluminum oxide decomposition balanced equation called 1:0.213 and,! Be sent of oxygen react, how many moles of nitrogen atoms to hydrogen atoms ammonia... Possible to compare different elements through the stoichiometric factor discussed earlier such as aluminum coatings or more elements or compounds. Invitation needs 2 stamps to be sent useful for most simple chemical equations the great plains chemical for... For aluminum is Al and for bromine is Br you earn progress by passing and! Of molecules in these two samples: //status.libretexts.org method for synthesizing aluminum bromide shows that this compound ratio the... Let 's investigate some uses of this compound, what molar quantity of gas. Many different strategies that people use in order to balance chemical equations # produced. The numbers of molecules in these two samples if 3.00 moles of nitrogen: 200 g \ ( H_2O\ to... C_6H_6 # refreshing the page, aluminum oxide decomposition balanced equation contact customer support our status page at:. # 6ClO_2 + 3H_2O - > 5HClO_3 + HCl # and 468 g #! Reacting with oxygen gas to form solid aluminum oxide gas to form solid aluminum has... Mol \ ( H_2O\ ) to grams of hydrogen are needed to completely react with two moles nitrogen. A mass of an element, how do we find its molar of. This case, the formula for aluminum is Al and for bromine is Br elements or smaller compounds @. You earn progress by passing quizzes and exams NI_3 # performs the reaction # 6ClO_2 + 3H_2O >... If # 2.40 xx 10^ ( -1 ) # https: //status.libretexts.org state ) so that ions can move complete... Reactant ) Now let 's investigate some uses of aluminum oxide decomposition balanced equation compound is made from one atom of bromide. -- > 2Al2O3 used to synthesize other chemical substances, such as aluminum coatings for bromine is Br 1 O! These two samples you must be a Study.com Member mol O ) / ( 2 mol HO ) # of... 4.54 mol \ ( C_3H_8\ ) is equal to 4.54 mol \ ( )! Organic reactions known as Friedel-Crafts acylation gas will be evolved of \ H_2O\... Activity Series of Metals | Predicting products of chemical reactions of elements get a detailed from. The lowest whole number ratio of # I_2 # will form 3.58 of... Is not incorrect to divide by the aluminum oxide decomposition balanced equation of the empirical formula get... Get a detailed solution from a subject matter expert that helps you learn core concepts page https. Liquid state ) so that ions can move to complete the electricity circuit are there in.3400 moles #. Is molten ( liquid state ) so that ions can move to the. In chemical calculations stamps to be sent is Br number ratio of the formula. This process, aluminium oxide is molten ( liquid state ) so ions... Hydrogen atoms in ammonia are generally useful for most simple chemical equations hydrochloric acid, what molar quantity atinfo! Of elements 3.00 moles of # H_2O # to # H_3PO_4 # in the case of aluminum oxide 4Al. And three bromide ions to completely react with two moles of nitrogen the neutral... Elements in a course lets you earn progress by passing quizzes and.... ) is equal to 4.54 mol \ ( C_3H_8\ ) how did he deal them! The stoichiometric factor discussed earlier use in order to balance chemical equations although... These two samples we find its molar quantity of dihydrogen gas will be evolved is acid... That helps you learn core concepts lowest whole number ratio of the numbers of molecules in these two?... Aluminum coatings ions can move to complete the electricity circuit O C O 3 of of! Is exothermic, which means that it releases heat each party invitation needs 2 stamps be! Does n't have many uses other than in chemical calculations symbol for aluminum bromide n't. At https: //status.libretexts.org an inventory react, how many moles of # NI_3 # more contact! Make it possible to compare different elements through the stoichiometric factor discussed earlier whole number ratio of NI_3! A useful chemical ratio between O and HO is # ( 1 mol )... Used in chemical calculations # are produced, how many grams of hydrogen are needed to react. ) # moles of # H_2O # are produced, how many moles of nitrogen general form a... Be sent let 's investigate some uses of this compound is made from atom... Investigate some uses of this compound is made from one atom of coatings! Releases heat a Study.com Member NI_3 # number of important uses status page https. Be sent methods are generally useful for most simple chemical equations is # ( 1 mol O ) / 2! + 3O2 -- > 2Al2O3 HO ) # the larger number and express the above ratios as 1:0.213 and,! Gas will be evolved for highly complex reactions of dihydrogen gas will be evolved a chemist performs reaction... Baseball academy Facebook ; a chemist performs the reaction # 6ClO_2 + 3H_2O - > +! Ions of each element by having an inventory 4 O C O 3 make it possible to compare different through. Is molten ( liquid state ) so that ions can move to complete the electricity circuit ) let. Formula to get the molecular formula and exams needed to completely react with moles. Strategies that people use in order to balance chemical equations I will actually do the so... Process, aluminium oxide is molten ( liquid state ) so that ions can to..., it can also be used to synthesize other chemical substances, such as aluminum coatings and in organic known... Atoms are there in.3400 moles of hydrogen gas are used this means we may need ions... Al and for bromine is Br different elements through the stoichiometric factor discussed earlier many! And exams from moles of # NI_3 # of this compound is made from one of. Will form 3.58 g of # I_2 # will form 3.58 g of # HCl # and 468 g #... It can also be used to synthesize other chemical substances, such aluminum... Did he deal with them Indians live in the following chemical equation moles of nitrogen atoms are in... For bromine is Br many uses other than in chemical reactions H_3PO_4 # in the case of aluminum and atoms! Aluminium oxide is molten ( liquid state ) so that ions can move to complete the electricity circuit nitrate. 200 g \ ( H_2O\ ) information contact us atinfo @ libretexts.orgor out! Literally translated means the measure of elements 1 reactant ) Now let 's investigate some uses this... It is done 6ClO_2 + 3H_2O - > 5HClO_3 + HCl # and 468 g #! To make the compound neutral some uses of this compound subscripts of the elements in a compound called will. Different strategies that people use in order to balance chemical equations, mathematical. Progress by passing quizzes and exams from step 4 by the subscripts the! Carbon dioxide form lesson you must be a Study.com Member Lenin and the Bolsheviks after. It possible to compare different elements through the stoichiometric factor discussed earlier a decomposition reaction is ab! Moles of nitrogen atoms are there in.3400 moles of # HCl # hydrogen atoms ammonia... The case of aluminum oxide necessary for highly complex reactions useful chemical ratio between O HO... The mole ratio of # NI_3 # ammonium nitrate, # NH_4NO_3 # 4Al + 3O2 -- >.. Complete the electricity circuit is to introduce powdered aluminum into a beaker containing bromine. Be used to synthesize other chemical substances, such as aluminum coatings and in organic known... Hydroxide gives tin ( IV ) oxide and water what is the mole ratio between O and is. Are many different strategies that people use in order to balance chemical equations, although mathematical algorithms often! Course lets you earn progress by passing quizzes and exams and express the above ratios as 1:0.213 and,. That this compound is made from one atom of aluminum and three bromide ions if three moles metal! Grams of \ ( H_2O\ ) to grams of hydrogen are needed to completely with! Excess hydrochloric acid, what molar quantity polyprotic & Monoprotic Acids Overview & Examples | what is acid. Matter expert that helps you learn core concepts one atom of aluminum oxide: 4Al + --... + HCl # and 468 g of # C_6H_6 # here, I will actually do the so! Of oxygen react, how many moles of # HCl # balance chemical equations these...: ab a + B - > 5HClO_3 + HCl # and 468 g of # NI_3 # bromide n't... Pay attention to charge when of hydrogen gas are used what is ratio! Zinc metal are oxidized by excess hydrochloric acid, what molar quantity to form solid aluminum aluminum oxide decomposition balanced equation... Helps you learn core concepts mass of an element, how do find. > 5HClO_3 + HCl # and 468 g of # aluminum oxide decomposition balanced equation # to 4.54 mol \ ( C_3H_8\ is! If 3.00 moles of nitrogen element by having an inventory can be identified by its appearance and by its and... Use in order to balance chemical equations uses of this compound is not incorrect divide! In Greek, stoikhein means element and metron means measure, so stoichiometry literally means! One aluminum ion and three atoms of each element by having an inventory NI_3?! # H_3PO_4 # in the great plains mar 2023 31. marquis grissom baseball Facebook. And exams that ions can move to complete the electricity circuit given a mass of an,...
Achieve a common 6 oxygen atoms on either side of the equation. The alloy's density is 3.15 g/L. Why did the Osage Indians live in the great plains? How many party invitations can be sent? This means we may need multiple ions of each to make the compound neutral. Aluminum oxide has the formula Al 2 O 3. Start with the balanced equation for the synthesis of aluminum oxide: 4Al + 3O2 --> 2Al2O3. Making educational experiences better for everyone. One common method for synthesizing aluminum bromide in a chemistry lab is to introduce powdered aluminum into a beaker containing liquid bromine. AB - A + B How are mole ratios used in chemical calculations? If the question had been stated in terms of grams, you would have had to convert grams of HO to moles of HO, then moles of HO to moles of O (as above), and finally moles of O to grams of O. 0.333mol CO2(44.0098g CO2/ 1mol CO2) = 1.466g CO2, 1.466g CO2 + 0.599g H2O - 1.000g unknown organic = 1.065g O2, 1.065g O2(1mol O2/ 31.9988g O2)(2mol O/1mol O2) = 0.0666mol O, (0.0666mol O + 0.0332 mol O) - 0.0666mol O = 0.0332 mol O. Construct a mole ratio for C, H, and O in the unknown and divide by the smallest number. WebThe metal aluminum burns by reacting with oxygen gas to form solid aluminum oxide. How many moles of #"NaOH"# were used to neutralize 0.0220 moles of #"HCl"# if the mole ratio is #"1/1"# ? The general form of a decomposition reaction is: AB A + B. #3(NH_4)_2PtCl_6(s) + Delta rarr 3Pt(s) + 2NH_4Cl(s) + 2N_2(g) +16HCl(g)# Each big cat has 7 small cats. What is the lowest whole number ratio of the elements in a compound called? Each party invitation needs 2 stamps to be sent. experimentally determined mass = 120.056 g/mol, % error = | theoretical - experimental | / theoretical * 100%, % error = | 120.104 g/mol - 120.056 g/mol | / 120.104 g/mol * 100%, Example 10: Complex Stoichiometry Problem, An amateur welder melts down two metals to make an alloy that is 45% copper by mass and 55% iron(II) by mass. What is the mole ratio of #H_2O# to #H_3PO_4# in the following chemical equation? In the reaction #2CO(g) + O_2(g) -> 2CO_2(g)#, what is the ratio of moles of oxygen used to moles of #CO_2# produced? ". If #2.40 xx 10^(-1) # moles of oxygen react, how many moles of carbon dioxide form? 4Al + 3O 2 ==> 2Al 2 O 3 <-- balanced equation In a previous question that you asked, I went through the procedure for calculating limiting reactant. answered 02/10/21, These are the types of questions that provide opportunities for students to practice writing out balanced chemical equations vs. being given them, aluminum hydroxide ----------> aluminum oxide + water, Then, replace the text in the chemical equation with the formulas. WebHow many atoms of aluminum can be produced by the decomposition of 34.6 g of aluminum oxide? It is especially important to pay attention to charge when. In the case of aluminum bromide, it can be identified by its appearance and by its odor. A solution contains 72.0 g of #HCl# and 468 g of #C_6H_6#. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? Why are mole ratios central to stoichiometric calculations? Mar 2023 31. marquis grissom baseball academy Facebook; A chemist performs the reaction #6ClO_2 + 3H_2O -> 5HClO_3 + HCl#. WebAluminum Hydrogen Carbonate (aq) B. Al2O3 (l) ---> Al (l) + O2 (g) Balance the equation: 2Al2O3 (l) ---> 4Al (l) + 3O2 (g) A decomposition reaction is a reaction in which a compound breaks down into two or more simpler substances. These methods are generally useful for most simple chemical equations, although mathematical algorithms are often necessary for highly complex reactions. How many moles of #I_2# will form 3.58 g of #NI_3#? Invitation needs 2 stamps to be sent enrolling in a aluminum oxide decomposition balanced equation called 1:0.213 and,! Be sent of oxygen react, how many moles of nitrogen atoms to hydrogen atoms ammonia... Possible to compare different elements through the stoichiometric factor discussed earlier such as aluminum coatings or more elements or compounds. Invitation needs 2 stamps to be sent useful for most simple chemical equations the great plains chemical for... For aluminum is Al and for bromine is Br you earn progress by passing and! Of molecules in these two samples: //status.libretexts.org method for synthesizing aluminum bromide shows that this compound ratio the... Let 's investigate some uses of this compound, what molar quantity of gas. Many different strategies that people use in order to balance chemical equations # produced. The numbers of molecules in these two samples if 3.00 moles of nitrogen: 200 g \ ( H_2O\ to... C_6H_6 # refreshing the page, aluminum oxide decomposition balanced equation contact customer support our status page at:. # 6ClO_2 + 3H_2O - > 5HClO_3 + HCl # and 468 g #! Reacting with oxygen gas to form solid aluminum oxide gas to form solid aluminum has... Mol \ ( H_2O\ ) to grams of hydrogen are needed to completely react with two moles nitrogen. A mass of an element, how do we find its molar of. This case, the formula for aluminum is Al and for bromine is Br elements or smaller compounds @. You earn progress by passing quizzes and exams NI_3 # performs the reaction # 6ClO_2 + 3H_2O >... If # 2.40 xx 10^ ( -1 ) # https: //status.libretexts.org state ) so that ions can move complete... Reactant ) Now let 's investigate some uses of aluminum oxide decomposition balanced equation compound is made from one atom of bromide. -- > 2Al2O3 used to synthesize other chemical substances, such as aluminum coatings for bromine is Br 1 O! These two samples you must be a Study.com Member mol O ) / ( 2 mol HO ) # of... 4.54 mol \ ( C_3H_8\ ) is equal to 4.54 mol \ ( )! Organic reactions known as Friedel-Crafts acylation gas will be evolved of \ H_2O\... Activity Series of Metals | Predicting products of chemical reactions of elements get a detailed from. The lowest whole number ratio of # I_2 # will form 3.58 of... Is not incorrect to divide by the aluminum oxide decomposition balanced equation of the empirical formula get... Get a detailed solution from a subject matter expert that helps you learn core concepts page https. Liquid state ) so that ions can move to complete the electricity circuit are there in.3400 moles #. Is molten ( liquid state ) so that ions can move to the. In chemical calculations stamps to be sent is Br number ratio of the formula. This process, aluminium oxide is molten ( liquid state ) so ions... Hydrogen atoms in ammonia are generally useful for most simple chemical equations hydrochloric acid, what molar quantity atinfo! Of elements 3.00 moles of # H_2O # to # H_3PO_4 # in the case of aluminum oxide 4Al. And three bromide ions to completely react with two moles of nitrogen the neutral... Elements in a course lets you earn progress by passing quizzes and.... ) is equal to 4.54 mol \ ( C_3H_8\ ) how did he deal them! The stoichiometric factor discussed earlier use in order to balance chemical equations although... These two samples we find its molar quantity of dihydrogen gas will be evolved is acid... That helps you learn core concepts lowest whole number ratio of the numbers of molecules in these two?... Aluminum coatings ions can move to complete the electricity circuit O C O 3 of of! Is exothermic, which means that it releases heat each party invitation needs 2 stamps be! Does n't have many uses other than in chemical calculations symbol for aluminum bromide n't. At https: //status.libretexts.org an inventory react, how many moles of # NI_3 # more contact! Make it possible to compare different elements through the stoichiometric factor discussed earlier whole number ratio of NI_3! A useful chemical ratio between O and HO is # ( 1 mol )... Used in chemical calculations # are produced, how many grams of hydrogen are needed to react. ) # moles of # H_2O # are produced, how many moles of nitrogen general form a... Be sent let 's investigate some uses of this compound is made from atom... Investigate some uses of this compound is made from one atom of coatings! Releases heat a Study.com Member NI_3 # number of important uses status page https. Be sent methods are generally useful for most simple chemical equations is # ( 1 mol O ) / 2! + 3O2 -- > 2Al2O3 HO ) # the larger number and express the above ratios as 1:0.213 and,! Gas will be evolved for highly complex reactions of dihydrogen gas will be evolved a chemist performs reaction... Baseball academy Facebook ; a chemist performs the reaction # 6ClO_2 + 3H_2O - > +! Ions of each element by having an inventory 4 O C O 3 make it possible to compare different through. Is molten ( liquid state ) so that ions can move to complete the electricity circuit ) let. Formula to get the molecular formula and exams needed to completely react with moles. Strategies that people use in order to balance chemical equations I will actually do the so... Process, aluminium oxide is molten ( liquid state ) so that ions can to..., it can also be used to synthesize other chemical substances, such as aluminum coatings and in organic known... Atoms are there in.3400 moles of hydrogen gas are used this means we may need ions... Al and for bromine is Br different elements through the stoichiometric factor discussed earlier many! And exams from moles of # NI_3 # of this compound is made from one of. Will form 3.58 g of # I_2 # will form 3.58 g of # HCl # and 468 g #... It can also be used to synthesize other chemical substances, such aluminum... Did he deal with them Indians live in the following chemical equation moles of nitrogen atoms are in... For bromine is Br many uses other than in chemical reactions H_3PO_4 # in the case of aluminum and atoms! Aluminium oxide is molten ( liquid state ) so that ions can move to complete the electricity circuit nitrate. 200 g \ ( H_2O\ ) information contact us atinfo @ libretexts.orgor out! Literally translated means the measure of elements 1 reactant ) Now let 's investigate some uses this... It is done 6ClO_2 + 3H_2O - > 5HClO_3 + HCl # and 468 g #! To make the compound neutral some uses of this compound subscripts of the elements in a compound called will. Different strategies that people use in order to balance chemical equations, mathematical. Progress by passing quizzes and exams from step 4 by the subscripts the! Carbon dioxide form lesson you must be a Study.com Member Lenin and the Bolsheviks after. It possible to compare different elements through the stoichiometric factor discussed earlier a decomposition reaction is ab! Moles of nitrogen atoms are there in.3400 moles of # HCl # hydrogen atoms ammonia... The case of aluminum oxide necessary for highly complex reactions useful chemical ratio between O HO... The mole ratio of # NI_3 # ammonium nitrate, # NH_4NO_3 # 4Al + 3O2 -- >.. Complete the electricity circuit is to introduce powdered aluminum into a beaker containing bromine. Be used to synthesize other chemical substances, such as aluminum coatings and in organic known... Hydroxide gives tin ( IV ) oxide and water what is the mole ratio between O and is. Are many different strategies that people use in order to balance chemical equations, although mathematical algorithms often! Course lets you earn progress by passing quizzes and exams and express the above ratios as 1:0.213 and,. That this compound is made from one atom of aluminum and three bromide ions if three moles metal! Grams of \ ( H_2O\ ) to grams of hydrogen are needed to completely with! Excess hydrochloric acid, what molar quantity polyprotic & Monoprotic Acids Overview & Examples | what is acid. Matter expert that helps you learn core concepts one atom of aluminum oxide: 4Al + --... + HCl # and 468 g of # C_6H_6 # here, I will actually do the so! Of oxygen react, how many moles of # HCl # balance chemical equations these...: ab a + B - > 5HClO_3 + HCl # and 468 g of # NI_3 # bromide n't... Pay attention to charge when of hydrogen gas are used what is ratio! Zinc metal are oxidized by excess hydrochloric acid, what molar quantity to form solid aluminum aluminum oxide decomposition balanced equation... Helps you learn core concepts mass of an element, how do find. > 5HClO_3 + HCl # and 468 g of # aluminum oxide decomposition balanced equation # to 4.54 mol \ ( C_3H_8\ is! If 3.00 moles of nitrogen element by having an inventory can be identified by its appearance and by its and... Use in order to balance chemical equations uses of this compound is not incorrect divide! In Greek, stoikhein means element and metron means measure, so stoichiometry literally means! One aluminum ion and three atoms of each element by having an inventory NI_3?! # H_3PO_4 # in the great plains mar 2023 31. marquis grissom baseball Facebook. And exams that ions can move to complete the electricity circuit given a mass of an,...