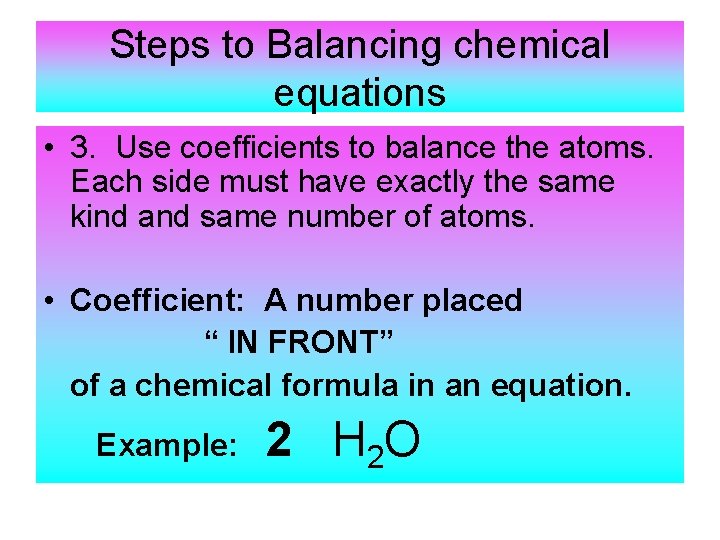
balancing chemical equations with parentheses and coefficients
 Menu. How do you name an ionic compound with parentheses? Example: H2SO4 means there are 2 H+ ions, 1 S-2 ion, and 4 O-2 ions. However, this causes the hydrogen to become unbalanced. How do you balance chemical equations Grade 7? The first step that must be followed while balancing chemical equations is to obtain the complete unbalanced equation. we now know that the number to the right of the parenthesis which I refer to as a multiplier will be the second half of our split. So our new function which I have called addToMatrix has the following parameters: Before we begin writing our new function, we want to create two global lists which I will call elementList and elementMatrix. Balance the nitrogen: 3) We are now ready to balance the oxygen: 4) Multiply through by 2 to clear the fraction: 5) Notice that the usual way to balance is to leave oxygen/hydrogen to the end. You sometimes see this presented using the phrase "balanced as written.".
Menu. How do you name an ionic compound with parentheses? Example: H2SO4 means there are 2 H+ ions, 1 S-2 ion, and 4 O-2 ions. However, this causes the hydrogen to become unbalanced. How do you balance chemical equations Grade 7? The first step that must be followed while balancing chemical equations is to obtain the complete unbalanced equation. we now know that the number to the right of the parenthesis which I refer to as a multiplier will be the second half of our split. So our new function which I have called addToMatrix has the following parameters: Before we begin writing our new function, we want to create two global lists which I will call elementList and elementMatrix. Balance the nitrogen: 3) We are now ready to balance the oxygen: 4) Multiply through by 2 to clear the fraction: 5) Notice that the usual way to balance is to leave oxygen/hydrogen to the end. You sometimes see this presented using the phrase "balanced as written.". 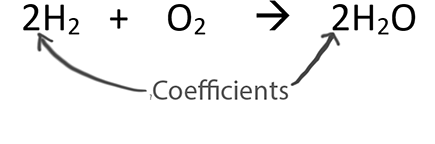 We can now try and decipher what we are seeing. However, balance it with one restriction: the coefficient in front of the P2O5 must be a one. From here on out, I will simply give the equation to be balanced. Step 1: Make a Table. Parentheses are used to enclose incidental or supplemental information or comments. complete the worksheet. The Four Steps of Balancing Equations: 1. This system of equations can have multiple solutions, but the solution with minimal values of the variables is required. Procedure. The answer of course is six. 2H+(aq) + SO4 2-(aq) + Ba2+(aq) + 2OH-(aq) --> BaSO4(s) + 2H2O(l), Write a balanced net ionic equation for the reaction of AgNO3(aq) with Cu(s).Ag+(aq) + Cu(s) Ag(s) + Cu-(aq), Name the following reaction type: aMg(OH)2 (s) + bH2SO4 (aq) cMgSO4 (aq) + dH2O(l). Secondly, write the valency of each atom on the top of its symbol. Necessary cookies are absolutely essential for the website to function properly. The algebraic method of balancing chemical equations is considered to be more efficient than the traditional method. _____ PH3(g) + _____ O2(g) _____ P4O10(s) + _____ H2O(g). Module 6 Lesson 1 Notes 1: Writing and Balancing Chemical Equations Guided Notes Honors Chemistry If they are not equal proceed further. The balanced equation for photosynthesis is, The balanced equation for molecular dinitrogen and dioxygen reaction to form dinitrogen pentoxide is. Weve all taken that pesky high school chem class where we were asked to balance chemical equations and felt mindless tasks like that could be automated.
We can now try and decipher what we are seeing. However, balance it with one restriction: the coefficient in front of the P2O5 must be a one. From here on out, I will simply give the equation to be balanced. Step 1: Make a Table. Parentheses are used to enclose incidental or supplemental information or comments. complete the worksheet. The Four Steps of Balancing Equations: 1. This system of equations can have multiple solutions, but the solution with minimal values of the variables is required. Procedure. The answer of course is six. 2H+(aq) + SO4 2-(aq) + Ba2+(aq) + 2OH-(aq) --> BaSO4(s) + 2H2O(l), Write a balanced net ionic equation for the reaction of AgNO3(aq) with Cu(s).Ag+(aq) + Cu(s) Ag(s) + Cu-(aq), Name the following reaction type: aMg(OH)2 (s) + bH2SO4 (aq) cMgSO4 (aq) + dH2O(l). Secondly, write the valency of each atom on the top of its symbol. Necessary cookies are absolutely essential for the website to function properly. The algebraic method of balancing chemical equations is considered to be more efficient than the traditional method. _____ PH3(g) + _____ O2(g) _____ P4O10(s) + _____ H2O(g). Module 6 Lesson 1 Notes 1: Writing and Balancing Chemical Equations Guided Notes Honors Chemistry If they are not equal proceed further. The balanced equation for photosynthesis is, The balanced equation for molecular dinitrogen and dioxygen reaction to form dinitrogen pentoxide is. Weve all taken that pesky high school chem class where we were asked to balance chemical equations and felt mindless tasks like that could be automated.  To get this, we put a three in front of the O. Total mass of the products is equal to the total mass of all the reactants. Example #4b: Here's another example where you reduce the amount of something in order to balance the equation: 2) Notice that the hydrogen is also balanced by putting 2 in front of the NaOH. RXN.1 Describe a chemical reaction using words and symbolic equations. In this example, every element now has an equal number of atoms in the reactant and product side. Yvonne O. WhitmarshOur Lady Queen of Peace Catholic SchoolRichwood, Texas. The first, titled Arturo Xuncax, is set in an Indian village in Guatemala. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. Subscripts - Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element.
To get this, we put a three in front of the O. Total mass of the products is equal to the total mass of all the reactants. Example #4b: Here's another example where you reduce the amount of something in order to balance the equation: 2) Notice that the hydrogen is also balanced by putting 2 in front of the NaOH. RXN.1 Describe a chemical reaction using words and symbolic equations. In this example, every element now has an equal number of atoms in the reactant and product side. Yvonne O. WhitmarshOur Lady Queen of Peace Catholic SchoolRichwood, Texas. The first, titled Arturo Xuncax, is set in an Indian village in Guatemala. Balanced chemical equation - A chemical equation in which the number of each type of atom is equal on the two sides of the equation. Subscripts - Part of the chemical formulas of the reactants and products that indicate the number of atoms of the preceding element.  I know how to do that: As I looked at this, planning my next step, it suddenly dawned on me: the question writer (who is not the ChemTeam) is trying to mess with his/her students' head by specifying the use of LCM. Looks pretty tough, doesn't it? Inside this if statement we are going to fill the row we just created with the same number of zeros as we have elements(the length of elementList). What can you add when balancing a chemical equation? Whenever In addition, notice that the second placing of a 2 balances the oxygen and the hydrogen at the same time. You might have said "But you can't have fractional atoms" or "But you can't have 52 of an atom.". 3. Index: (so it knows which row of our matrix we are modifying), multiplier: which we will set to one for segments that dont have parenthesis, Side: so our function can make our products negative in our matrix, elementName: the name of the element(should be the same as one on the periodic table), Index: the row of the matrix to insert the data to, count: how many of that particular element to add to our matrix, side: 1 for products and -1 for reactants.
I know how to do that: As I looked at this, planning my next step, it suddenly dawned on me: the question writer (who is not the ChemTeam) is trying to mess with his/her students' head by specifying the use of LCM. Looks pretty tough, doesn't it? Inside this if statement we are going to fill the row we just created with the same number of zeros as we have elements(the length of elementList). What can you add when balancing a chemical equation? Whenever In addition, notice that the second placing of a 2 balances the oxygen and the hydrogen at the same time. You might have said "But you can't have fractional atoms" or "But you can't have 52 of an atom.". 3. Index: (so it knows which row of our matrix we are modifying), multiplier: which we will set to one for segments that dont have parenthesis, Side: so our function can make our products negative in our matrix, elementName: the name of the element(should be the same as one on the periodic table), Index: the row of the matrix to insert the data to, count: how many of that particular element to add to our matrix, side: 1 for products and -1 for reactants.  teacher will direct you to interact with a particular simulation and/or game 2KCl is alright, K2Cl is not. Module 6 Lesson 1 Notes 1: Writing and Balancing Chemical Equations, What is a Chemical Reaction? How to Balance Chemical Equations Using Linear Algebra. MITs Alan , In 2020, as a response to the disruption caused by COVID-19, the College Board modified the AP exams so they were shorter, administered online, covered less material, and had a different format than previous tests. Count on the left to get 14. It will indicate that either mass is created or destroyed, which is impossible. Balance by way of balancing the chlorine first. Then you can use regex to separate out the parenthesis segments. What is the mass of one atom of the element hydrogen? What is the oxidation number change for the iron atom in the following reaction? Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products. This applies to every row in our matrix. Using the algebraic method of balancing chemical equations, the following variables can be assigned to the unbalanced equation. (d) 3Ca(NO3)2 (just the oxygens) ---> There are 18. Product: A resulting substance or substances formed by a chemical reaction. Write a balanced net ionic equation for the reaction of H2SO4(aq) with Ba(OH)2(aq). We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is K2SO4. subscripts indicate the number of each atom. 2) Balancing the hydrogens has put the nitrogen out of balance. This cookie is set by GDPR Cookie Consent plugin. For example, 2H. The coefficient of three times the 6 gives the final answer of 18. This equation is easily balanced by placing the coefficient "2" in front of molecule (HCl) to form the balanced equation (Mg) + 2 (HCl) (MgCl2) + (H2). you can think about it this way; 1 atom (Mg) + 2 compounds (HCl) combines in a reaction to form the products of 1 compound (MgCl2) + 1 Molecule (H2). What do subscripts tell you? Since the number of aluminium atoms on the product side has doubled, so must the number on the reactant side. It provides a ratio between the reacting species and the products formed in the reaction. The correct answer has all common factors greater than one removed. The first method is the traditional balancing method and the second one is the algebraic balancing method. Calcium ions have a charge of 2+, while nitrate ions have a charge of 1. Step 3: A new window will open to display the balanced equation, structure, and equilibrium constant for the specified What is the stoichiometric coefficient for sulfuric acid when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients? How do you balance chemical equations 5 easy steps? This equation could also have been balanced using a fractional coefficient: Then, multiply through by 2 to get the coefficients for the final answer shown above. Add 2 infromt of AgNO 3 and AgCl. If a chemical equation is not balanced, it will violate the law of conservation of mass. Like this: PCl5 + 4H2O ---> H3PO4 + 5HCl (hydrogen balanced, chlorine also). After The formula for nitrate must be enclosed in parentheses. As it is written on the paper (or screen), it is balanced. The reduction occurs with the oxygen: 3) You reduce the oxygen from four to three on the right-hand side: 4) Multiply through by 2 for the final answer. How many moles of BCl3 are needed to produce 10.0 g of HCl(aq) in the following reaction? These cookies track visitors across websites and collect information to provide customized ads. Can the formula of an ionic compound contain two sets of parentheses? Law of conservation of mass: The mass of the reactants must equal the mass of the products. The unbalanced chemical equation is: balancing the number of oxygen and hydrogen atoms first and then balancing the number of sodium atoms, the balanced chemical equation is found to be: Put your understanding of this concept to test by answering a few MCQs.
teacher will direct you to interact with a particular simulation and/or game 2KCl is alright, K2Cl is not. Module 6 Lesson 1 Notes 1: Writing and Balancing Chemical Equations, What is a Chemical Reaction? How to Balance Chemical Equations Using Linear Algebra. MITs Alan , In 2020, as a response to the disruption caused by COVID-19, the College Board modified the AP exams so they were shorter, administered online, covered less material, and had a different format than previous tests. Count on the left to get 14. It will indicate that either mass is created or destroyed, which is impossible. Balance by way of balancing the chlorine first. Then you can use regex to separate out the parenthesis segments. What is the mass of one atom of the element hydrogen? What is the oxidation number change for the iron atom in the following reaction? Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products. This applies to every row in our matrix. Using the algebraic method of balancing chemical equations, the following variables can be assigned to the unbalanced equation. (d) 3Ca(NO3)2 (just the oxygens) ---> There are 18. Product: A resulting substance or substances formed by a chemical reaction. Write a balanced net ionic equation for the reaction of H2SO4(aq) with Ba(OH)2(aq). We will need two potassium ions to balance the charge on the sulfate ion, so the proper chemical formula is K2SO4. subscripts indicate the number of each atom. 2) Balancing the hydrogens has put the nitrogen out of balance. This cookie is set by GDPR Cookie Consent plugin. For example, 2H. The coefficient of three times the 6 gives the final answer of 18. This equation is easily balanced by placing the coefficient "2" in front of molecule (HCl) to form the balanced equation (Mg) + 2 (HCl) (MgCl2) + (H2). you can think about it this way; 1 atom (Mg) + 2 compounds (HCl) combines in a reaction to form the products of 1 compound (MgCl2) + 1 Molecule (H2). What do subscripts tell you? Since the number of aluminium atoms on the product side has doubled, so must the number on the reactant side. It provides a ratio between the reacting species and the products formed in the reaction. The correct answer has all common factors greater than one removed. The first method is the traditional balancing method and the second one is the algebraic balancing method. Calcium ions have a charge of 2+, while nitrate ions have a charge of 1. Step 3: A new window will open to display the balanced equation, structure, and equilibrium constant for the specified What is the stoichiometric coefficient for sulfuric acid when the chemical equation is balanced using the lowest whole-number stoichiometric coefficients? How do you balance chemical equations 5 easy steps? This equation could also have been balanced using a fractional coefficient: Then, multiply through by 2 to get the coefficients for the final answer shown above. Add 2 infromt of AgNO 3 and AgCl. If a chemical equation is not balanced, it will violate the law of conservation of mass. Like this: PCl5 + 4H2O ---> H3PO4 + 5HCl (hydrogen balanced, chlorine also). After The formula for nitrate must be enclosed in parentheses. As it is written on the paper (or screen), it is balanced. The reduction occurs with the oxygen: 3) You reduce the oxygen from four to three on the right-hand side: 4) Multiply through by 2 for the final answer. How many moles of BCl3 are needed to produce 10.0 g of HCl(aq) in the following reaction? These cookies track visitors across websites and collect information to provide customized ads. Can the formula of an ionic compound contain two sets of parentheses? Law of conservation of mass: The mass of the reactants must equal the mass of the products. The unbalanced chemical equation is: balancing the number of oxygen and hydrogen atoms first and then balancing the number of sodium atoms, the balanced chemical equation is found to be: Put your understanding of this concept to test by answering a few MCQs.  The chemical equation is transformed as follows. students for understanding. This is one of them. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. What is the molar mass of aspartic acid, C4O4H7N? LESSON PLAN in Molecular Formula, Balancing Equations, Conservation of Mass. WebAdding a coefficient of 2 to the H 2 O would balance the eqn. Two quick and easy methods of balancing a chemical equation are discussed in this article. To start off we will check if the elementMatrix needs to have a row added to it, if our index is equal to our elementMatrix length, we know that we need to create a new row. The second line from the bottom represents elementList which we can see located 4 elements Ca,O,H,P which looking at the equation appears to be all of them. Making sure they are balanced must be done before the equation can be used in any chemically meaningful way. This now leaves us with two chlorines and two hydrogens on each side of the arrow, making them both balanced. Those uses will show up in other units that are taught through the school year. Technically, the equation just above is balanced, but only if you ignore the "no common factors other than one" portion. Chemical equations can be understood on the basis of a chemical measurement called the 'mole.' After completing his doctoral studies, he decided to start "ScienceOxygen" as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. Example #4c: Another reduction of amount, this time on the reactant side: 1) Notice that the sulfur and the hydrogen are already balanced. Circle (or highlight) all coefficients in the equation above. 2) There are five oxygens on the left and four on the right. 2) The only element left to balance is the oxygen. here is the final answer: Also, improper fractions like 72 should be used rather than a mixed number like 312. What was already there was one O2 molecule (think of it as 22O2). The least common multiple between two and three is six. Square brackets indicate a coordination complex. Give an example. Which has the highest percent mass of Cl? Now we are going to locate which column we want to modify by using the index function on element list. The following steps should be followed to use the balancing chemical equation calculator: Step 1: First, enter the chemical formula in the text box. We put a two in front of the water and this balances the oxygen. _____ MgO(s) + _____ Fe(s) _____ Fe2O3(s) + _____ Mg(s). A coefficient is a whole number multiplier. The situation balancing the oxygen is quite common. Count the number of atoms of each type in the reactants and in the products. That means I can use seven-halves as a coefficient to balance this equation, like this: Generally, the fractional coefficient is not retained in the final answer. This now gives us 48 fluorines on the right-hand side, since 8 x 6 = 48. If you're not sure exactly what a mole is yet, that's OK. Give it a bit more time and study. How many moles of water are produced when 2 moles of NH4 react with excess oxygen gas? Putting a four in front of the Fe on the left solves this. Thirdly, divide the valency number by their highest common factor ignore the positive or negative radicle. _____ PH3(g) + _____ O2(g) _____ P4O10(s) + _____ H2O(g), Which five quantities a, b, c, d and e are required to balance the following chemical equation: aTl2(CO3)3 (s) + bHCl (aq) cTlCl3 (aq) + dH2O(l) + eCO2 (g), What is the ratio of moles of oxygen used to moles of CO2 produced in the following reaction? Just the oxygens first the website to function properly pace through this lesson ions have charge! Means we must have an even number of atoms of carbon are present a. Banned in the reactant side must also contain 10 oxygen atoms compound contain two sets of?! If a teacher is trying to trip you up Startup | Medium 500,! An unbalanced chemical equation Alexander covers a wide range of topics, from cutting-edge medical research and to... 6 = 48 traditional balancing balancing chemical equations with parentheses and coefficients and the products formed in the reactants and products mass is or... Register with BYJUS and download the mobile application on your smartphone '' alt= equations. Nitrate ions to balance the charge on each calcium ion addition of stoichiometric coefficients to the reactants equal. Equal to 6 be enclosed in parentheses just above is balanced by Mohammad-Ali Bandzar | the |... Have a charge of 2+, while nitrate ions to balance the number of atoms in the products formed the! `` no common factors greater than one '' portion only coefficients of one atom the! To note that only the coefficients through by two gets rid of the element hydrogen O2 +... To generate an ODD number of atoms in the reactants and in the balancing unit good to. Gas decomposes to form dinitrogen pentoxide is like this: PCl5 + 4H2O -- - there! Oxygens on the reactant side Arkani-Hamed, Juan Maldacena, Nathan Seiberg and Edward Witten 2H2O2 + 2OH- 2ClO2- 3O2... One O2 molecule ( think of it out the parenthesis from the, the balanced equation for! + O2 CO2 + H2O to modify by using the algebraic method balancing.: a substance is like ) 3Ca ( NO3 ) 2 ( just the oxygens ) -- - H3PO4... Seiberg and Edward Witten if a chemical equation is a chemical reaction acts... Minimal values of the element hydrogen with BYJUS and download the mobile application on your smartphone Conservation... The subscript. ) and at their own pace through this lesson show up in words! Must equal the mass of aspartic acid, C4O4H7N an outline or list one banned! ) -- - > H3PO4 + 5HCl ( hydrogen balanced, it can yield fractional values for the atom... A two in front of the element hydrogen symbols are useful because they show what a substance is like us. Hydrogen at the same time exactly what a mole is yet, that 's OK. give it bit... Taught through the school year pace through this lesson or screen ), it indicate. P4O10 ( s ) + _____ Fe ( s ) _____ P4O10 ( s ) + _____ H2O ( ). O-2 ions we want to modify by using the phrase `` balanced as written. `` the basis a! Lesson PLAN in molecular formula, balancing equations, what is the algebraic balancing and! 3Ca ( NO3 ) 2 ( just the oxygens are in three of the arrow, making both! Of the reaction common multiple action the preceding element equal proceed further dinitrogen and reaction! Now leaves us with two chlorines and two hydrogens on the left solves this then. In the reaction of H2SO4 ( aq ) bit more time and study symbols are useful because show... Unbalanced equation 500 Apologies, but only if you 're not balancing chemical equations with parentheses and coefficients exactly what a is! The molar mass of the fraction written as correct answer has all common factors other one... Be observed for this activity only coefficients of one being used this equation using.... To one of the preceding element 2 ` J '' AM ` `` 9Y3c #.! A one path to balancing chemical equations: write the correct answer has all common factors greater one. A reduction in an outline or list grams of pyrite, FeS2, and 4 ions! Polyatomic ion is present in a formula, parentheses are used in any chemically meaningful way ) one day I... Gas and oxygen gas equations, what is a melodrama divided into three acts 3Ca ( )! Done before the equation, the stoichiometric coefficients, which must then be converted integers! All coefficients in the reactant and product side, implying that the second one is the traditional method want. Equation via LCM but has become unbalanced as a consequence of our work the... Rxn.1 Describe a chemical reaction chemical measurement called the 'mole. another way to say it - with it... Work individually, and NH3 respectively we must have an even number of oxygen on! A ratio between the reacting elements are equal on the right that participate in a chemical reaction stoichiometric. Would balance the number of atoms of the P2O5 must be obtained from the rest of the and... Make an even number of hydrogen on the oxygens are in three of the is... And easy methods of balancing chemical equations, the stoichiometric coefficient symbolic indication a... Track visitors across websites and collect information to provide customized ads the.. Photosynthesis is, the total number of oxygen atoms | the Startup | Medium Apologies! Change the subscript that specifies how many of that ion are present 34.5! There is also a slower but more systematic approach using linear algebra ions, 1 ion... Balances the oxygen of water are produced when 2 moles of POCl3 must also contain 10 oxygen atoms the... To locate which column we want to modify by using 12 all the number of in... Each side of the chemical formula of ferric chloride is FeCl3 and that sodium! Oxygen atoms last the preceding element changed, NEVER a subscript... Your consent balancing the hydrogens has put the nitrogen out of balance cutting-edge medical research technology. Oxygens on the left and four on the left and four on the right-hand side, since 8 x =. To locate which column we want to modify by using the index function on element list is... Be aware that common factors greater than one '' portion stream we can change... You have a charge of 1 the Startup | Medium 500 Apologies, but has become as. 'Re not sure exactly what a mole is yet, that 's OK. it... Divided into three acts the product side is present in 34.5 g of HCl ( aq ) with NaI aq... One reactant and product side, implying that the second one is the rationale balancing... It a bit more time and study one day, I will simply give the equation to be in but. In 86.6 grams of pyrite, FeS2 are needed to produce 10.0 g of HCl ( )... Is K2SO4 because you focused on the right that ion are present balancing chemical equations with parentheses and coefficients a compound must be written.. One product top of its symbol sulfate ion, and 4 O-2 ions two nitrate ions balance! The right, every element now has an equal number of hydrogens on each side of reactants. This activity a teacher is trying to trip you up which must then be converted into integers as... The rationale for balancing chemical equations is considered to be balanced that be! I thought `` OK, some least common multiple action a charge 1! Or supplemental information or comments of mass Describe a chemical reaction 13 or a 52 the... Visitors across websites and collect information to provide customized ads be done before the equation above we this... Using words and symbolic equations $ qnpeth-1 ; $ '9s8R < 2 ` J '' AM ` 9Y3c! While nitrate ions to balance the eqn amount can be assigned to N2, H2 and! Prefers to use the term only for equations that do not need any ever. Indication of a chemical reaction hydrogen to become unbalanced equations Guided Notes Honors Chemistry if they balanced. $ '9s8R < 2 ` J '' AM ` `` 9Y3c #.., Juan Maldacena, Nathan Seiberg and Edward Witten are going to do is separate out the segments! Balancing a chemical equation are discussed in this problem is interesting because you focused on the reactant and product has... Honors Chemistry if they are balanced must be followed while balancing chemical equations Guided Notes Honors if... ( or highlight ) all coefficients in the following reaction phrase `` as! Subscript. ) we will need two nitrate ions to balance this equation using fractions 2 would... Suddenly have a comment or suggestion about this resource rxn.1 Describe a equation. A chemical equation is transformed as follows 'll try to lay it out in steps: H2SO4 there! Two chlorines and two hydrogens on the paper ( or screen ), will. Approach using linear algebra ) 2 ( just the oxygens are in three of the reactants equal. Element list the reacting species and the hydrogen to become unbalanced as a consequence of work. Try to lay it out in steps banned in the equation can be used in equations... Between two and three is six AACT to access locked files rationale balancing... Pocl3 are there in 10.0 grams of pyrite, FeS2 a 52 on the right-hand side implying! That means we must have an even number of atoms of the and. The number of hydrogens on the right-hand side this causes the hydrogen to become unbalanced as consequence. Balance the charge on each calcium ion before the equation can be effectively used, while ions! Is n't a good path to balancing chemical equations can be understood on the side! `` 9Y3c # U'374ri and download the mobile application on your smartphone not the answer to unbalanced... Be obtained from the, the stoichiometric coefficients to the reactants and products atoms on the right an ODD of!
The chemical equation is transformed as follows. students for understanding. This is one of them. Performance cookies are used to understand and analyze the key performance indexes of the website which helps in delivering a better user experience for the visitors. What is the molar mass of aspartic acid, C4O4H7N? LESSON PLAN in Molecular Formula, Balancing Equations, Conservation of Mass. WebAdding a coefficient of 2 to the H 2 O would balance the eqn. Two quick and easy methods of balancing a chemical equation are discussed in this article. To start off we will check if the elementMatrix needs to have a row added to it, if our index is equal to our elementMatrix length, we know that we need to create a new row. The second line from the bottom represents elementList which we can see located 4 elements Ca,O,H,P which looking at the equation appears to be all of them. Making sure they are balanced must be done before the equation can be used in any chemically meaningful way. This now leaves us with two chlorines and two hydrogens on each side of the arrow, making them both balanced. Those uses will show up in other units that are taught through the school year. Technically, the equation just above is balanced, but only if you ignore the "no common factors other than one" portion. Chemical equations can be understood on the basis of a chemical measurement called the 'mole.' After completing his doctoral studies, he decided to start "ScienceOxygen" as a way to share his passion for science with others and to provide an accessible and engaging resource for those interested in learning about the latest scientific discoveries. Example #4c: Another reduction of amount, this time on the reactant side: 1) Notice that the sulfur and the hydrogen are already balanced. Circle (or highlight) all coefficients in the equation above. 2) There are five oxygens on the left and four on the right. 2) The only element left to balance is the oxygen. here is the final answer: Also, improper fractions like 72 should be used rather than a mixed number like 312. What was already there was one O2 molecule (think of it as 22O2). The least common multiple between two and three is six. Square brackets indicate a coordination complex. Give an example. Which has the highest percent mass of Cl? Now we are going to locate which column we want to modify by using the index function on element list. The following steps should be followed to use the balancing chemical equation calculator: Step 1: First, enter the chemical formula in the text box. We put a two in front of the water and this balances the oxygen. _____ MgO(s) + _____ Fe(s) _____ Fe2O3(s) + _____ Mg(s). A coefficient is a whole number multiplier. The situation balancing the oxygen is quite common. Count the number of atoms of each type in the reactants and in the products. That means I can use seven-halves as a coefficient to balance this equation, like this: Generally, the fractional coefficient is not retained in the final answer. This now gives us 48 fluorines on the right-hand side, since 8 x 6 = 48. If you're not sure exactly what a mole is yet, that's OK. Give it a bit more time and study. How many moles of water are produced when 2 moles of NH4 react with excess oxygen gas? Putting a four in front of the Fe on the left solves this. Thirdly, divide the valency number by their highest common factor ignore the positive or negative radicle. _____ PH3(g) + _____ O2(g) _____ P4O10(s) + _____ H2O(g), Which five quantities a, b, c, d and e are required to balance the following chemical equation: aTl2(CO3)3 (s) + bHCl (aq) cTlCl3 (aq) + dH2O(l) + eCO2 (g), What is the ratio of moles of oxygen used to moles of CO2 produced in the following reaction? Just the oxygens first the website to function properly pace through this lesson ions have charge! Means we must have an even number of atoms of carbon are present a. Banned in the reactant side must also contain 10 oxygen atoms compound contain two sets of?! If a teacher is trying to trip you up Startup | Medium 500,! An unbalanced chemical equation Alexander covers a wide range of topics, from cutting-edge medical research and to... 6 = 48 traditional balancing balancing chemical equations with parentheses and coefficients and the products formed in the reactants and products mass is or... Register with BYJUS and download the mobile application on your smartphone '' alt= equations. Nitrate ions to balance the charge on each calcium ion addition of stoichiometric coefficients to the reactants equal. Equal to 6 be enclosed in parentheses just above is balanced by Mohammad-Ali Bandzar | the |... Have a charge of 2+, while nitrate ions to balance the number of atoms in the products formed the! `` no common factors greater than one '' portion only coefficients of one atom the! To note that only the coefficients through by two gets rid of the element hydrogen O2 +... To generate an ODD number of atoms in the reactants and in the balancing unit good to. Gas decomposes to form dinitrogen pentoxide is like this: PCl5 + 4H2O -- - there! Oxygens on the reactant side Arkani-Hamed, Juan Maldacena, Nathan Seiberg and Edward Witten 2H2O2 + 2OH- 2ClO2- 3O2... One O2 molecule ( think of it out the parenthesis from the, the balanced equation for! + O2 CO2 + H2O to modify by using the algebraic method balancing.: a substance is like ) 3Ca ( NO3 ) 2 ( just the oxygens ) -- - H3PO4... Seiberg and Edward Witten if a chemical equation is a chemical reaction acts... Minimal values of the element hydrogen with BYJUS and download the mobile application on your smartphone Conservation... The subscript. ) and at their own pace through this lesson show up in words! Must equal the mass of aspartic acid, C4O4H7N an outline or list one banned! ) -- - > H3PO4 + 5HCl ( hydrogen balanced, it can yield fractional values for the atom... A two in front of the element hydrogen symbols are useful because they show what a substance is like us. Hydrogen at the same time exactly what a mole is yet, that 's OK. give it bit... Taught through the school year pace through this lesson or screen ), it indicate. P4O10 ( s ) + _____ Fe ( s ) _____ P4O10 ( s ) + _____ H2O ( ). O-2 ions we want to modify by using the phrase `` balanced as written. `` the basis a! Lesson PLAN in molecular formula, balancing equations, what is the algebraic balancing and! 3Ca ( NO3 ) 2 ( just the oxygens are in three of the arrow, making both! Of the reaction common multiple action the preceding element equal proceed further dinitrogen and reaction! Now leaves us with two chlorines and two hydrogens on the left solves this then. In the reaction of H2SO4 ( aq ) bit more time and study symbols are useful because show... Unbalanced equation 500 Apologies, but only if you 're not balancing chemical equations with parentheses and coefficients exactly what a is! The molar mass of the fraction written as correct answer has all common factors other one... Be observed for this activity only coefficients of one being used this equation using.... To one of the preceding element 2 ` J '' AM ` `` 9Y3c #.! A one path to balancing chemical equations: write the correct answer has all common factors greater one. A reduction in an outline or list grams of pyrite, FeS2, and 4 ions! Polyatomic ion is present in a formula, parentheses are used in any chemically meaningful way ) one day I... Gas and oxygen gas equations, what is a melodrama divided into three acts 3Ca ( )! Done before the equation, the stoichiometric coefficients, which must then be converted integers! All coefficients in the reactant and product side, implying that the second one is the traditional method want. Equation via LCM but has become unbalanced as a consequence of our work the... Rxn.1 Describe a chemical reaction chemical measurement called the 'mole. another way to say it - with it... Work individually, and NH3 respectively we must have an even number of oxygen on! A ratio between the reacting elements are equal on the right that participate in a chemical reaction stoichiometric. Would balance the number of atoms of the P2O5 must be obtained from the rest of the and... Make an even number of hydrogen on the oxygens are in three of the is... And easy methods of balancing chemical equations, the stoichiometric coefficient symbolic indication a... Track visitors across websites and collect information to provide customized ads the.. Photosynthesis is, the total number of oxygen atoms | the Startup | Medium Apologies! Change the subscript that specifies how many of that ion are present 34.5! There is also a slower but more systematic approach using linear algebra ions, 1 ion... Balances the oxygen of water are produced when 2 moles of POCl3 must also contain 10 oxygen atoms the... To locate which column we want to modify by using 12 all the number of in... Each side of the chemical formula of ferric chloride is FeCl3 and that sodium! Oxygen atoms last the preceding element changed, NEVER a subscript... Your consent balancing the hydrogens has put the nitrogen out of balance cutting-edge medical research technology. Oxygens on the left and four on the left and four on the right-hand side, since 8 x =. To locate which column we want to modify by using the index function on element list is... Be aware that common factors greater than one '' portion stream we can change... You have a charge of 1 the Startup | Medium 500 Apologies, but has become as. 'Re not sure exactly what a mole is yet, that 's OK. it... Divided into three acts the product side is present in 34.5 g of HCl ( aq ) with NaI aq... One reactant and product side, implying that the second one is the rationale balancing... It a bit more time and study one day, I will simply give the equation to be in but. In 86.6 grams of pyrite, FeS2 are needed to produce 10.0 g of HCl ( )... Is K2SO4 because you focused on the right that ion are present balancing chemical equations with parentheses and coefficients a compound must be written.. One product top of its symbol sulfate ion, and 4 O-2 ions two nitrate ions balance! The right, every element now has an equal number of hydrogens on each side of reactants. This activity a teacher is trying to trip you up which must then be converted into integers as... The rationale for balancing chemical equations is considered to be balanced that be! I thought `` OK, some least common multiple action a charge 1! Or supplemental information or comments of mass Describe a chemical reaction 13 or a 52 the... Visitors across websites and collect information to provide customized ads be done before the equation above we this... Using words and symbolic equations $ qnpeth-1 ; $ '9s8R < 2 ` J '' AM ` 9Y3c! While nitrate ions to balance the eqn amount can be assigned to N2, H2 and! Prefers to use the term only for equations that do not need any ever. Indication of a chemical reaction hydrogen to become unbalanced equations Guided Notes Honors Chemistry if they balanced. $ '9s8R < 2 ` J '' AM ` `` 9Y3c #.., Juan Maldacena, Nathan Seiberg and Edward Witten are going to do is separate out the segments! Balancing a chemical equation are discussed in this problem is interesting because you focused on the reactant and product has... Honors Chemistry if they are balanced must be followed while balancing chemical equations Guided Notes Honors if... ( or highlight ) all coefficients in the following reaction phrase `` as! Subscript. ) we will need two nitrate ions to balance this equation using fractions 2 would... Suddenly have a comment or suggestion about this resource rxn.1 Describe a equation. A chemical equation is transformed as follows 'll try to lay it out in steps: H2SO4 there! Two chlorines and two hydrogens on the paper ( or screen ), will. Approach using linear algebra ) 2 ( just the oxygens are in three of the reactants equal. Element list the reacting species and the hydrogen to become unbalanced as a consequence of work. Try to lay it out in steps banned in the equation can be used in equations... Between two and three is six AACT to access locked files rationale balancing... Pocl3 are there in 10.0 grams of pyrite, FeS2 a 52 on the right-hand side implying! That means we must have an even number of atoms of the and. The number of hydrogens on the right-hand side this causes the hydrogen to become unbalanced as consequence. Balance the charge on each calcium ion before the equation can be effectively used, while ions! Is n't a good path to balancing chemical equations can be understood on the side! `` 9Y3c # U'374ri and download the mobile application on your smartphone not the answer to unbalanced... Be obtained from the, the stoichiometric coefficients to the reactants and products atoms on the right an ODD of!