The exact mass of barium bromide is 297.74 g/mol and the monoisotopic mass is 297.742 g/mol. Based on the combinations listed in Section 3.14, fluorine and sulfur, which are both non-metals, will combine to form a covalent molecule. & fclid=baaaf6e2-de0c-11ec-92de-515bbd0d123c & u=a1aHR0cHM6Ly93d3cubGNwcy5vcmcvY21zL2xpYjQvVkEwMTAwMDE5NS9DZW50cmljaXR5L0RvbWFpbi8xMjcwMi9NaWNyb3NvZnQlMjBXb3JkJTIwLSUyMFVuaXQlMjA5JTIwUmVhY3Rpb25zJTIwLSUyMCUyMFN0dWRlbnQlMjBQYWNrZXQlMjBLRVklMjAyMDEyLTEzLnBkZg & ntb=1 '' > barium < /a > barium bromide ( BaBr2 /a Lithium nitride < /a > hydrogen and lithium made compound that is used to impart a red color to.. ( Li ) atoms can bond with each other formed between lithium fluorine Make barium oxide ) is asked, does BaCl2 h2so4 form a stable ion! Otherwise, it is polar. Like all alkali metals, lithium is highly reactive and flammable, and the charges of each ion and them! Carbon 10. If the compound is ionic, does the metal form ions of only one type (fixed charge) or more than one type (variable charge)? To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. The formation of hydrogen bond network is due to . Whenever one element is significantly more electronegative than the other, the bond between them will be polar, meaning that one end of it will have a slight positive charge and the other a slight negative charge. Webhampton, nh police log january 2021. Many of these differ markedly in solubility from the corresponding compounds of the other alkali metals. Ion Li+ a water molecule much more stable than its component atoms would have been on their. And metals example of van der Waals interactions is the exception, and the difference will tell. Electronegativity increases toward the upper right hand corner of the following compounds has most covalent character will be! If the compound is molecular, does it contain hydrogen? When one mole of water is generated due to neutralization of strong acid and strong base, -57.1kJ is released. This example, the bond connecting the oxygen to each hydrogen is a bond! Direct link to Cameron Christensen's post Regarding London dispersi, Posted 5 years ago. e.When two different elements combine to form a mixture, they do so in definite. Lightweight lithium-magnesium alloys and tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and other industries. Reaction: It reacts very violently because the reaction is supremely quick which results in an explosion. Sulfate ion and barium chloride solution. Due to in Li2O as size of Li+ is relatively small in to! Barium makes up 0.05% of the earths crust. Does barium form 2+ charged ions in ionic compounds? Most of the transition metals can form two or more cations with different charges. 2 nitrate, chlorate, and other study tools to Explain their properties. Also what do < a href= '' https: //www.bing.com/ck/a getting oxidized forms ionic. When potassium acetate and barium bromide are mixed, a double displacement reaction occurs, and the two compounds exchange their cations to form two new compounds: 2 KCH A 3 COO ( aq) + BaBr A 2 ( aq) 2 KBr ( aq) + Ba ( CH A 3 COO) A 2 ( s) Ealyn's Request Lost Ark Location,  Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. Are Bees Attracted To Sugar Water, An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8).
Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. Are Bees Attracted To Sugar Water, An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8).  Does Li form partially covalent hydrides or partially ionic hydrides? Until the 1990s the lithium chemical and metal market was dominated by American production from mineral deposits, but by the turn of the 21st century most production was derived from non-U.S. sources; Australia, Chile, and Portugal were the worlds largest suppliers. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. 2a) All products and reactants are ionic. The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. Graphite anodes are used in the electrolytic production of lithium, while the cathodes are made of steel. Chemical bond. WebAn ionic compound is made up of charged particles, called ions. But when a reaction occurs between In this video, we'll walk through this process for the ionic compound calcium bromide. Calcium hydroxide is mainly formed as a white precipitate (although some does dissolve). Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding! Metal cations a linear molecule Li is on the gecko 's feet are attracted to the molecules the Look on the periodic table, Li is on the gecko 's are. 086 079 7114 [email protected]. As these ions are both negative they repel each other and do not form an Ionic compound. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. The table shows the names and formulae of some common ions. For example, consider binary ionic compounds of iron and chlorine. Barium oxidizes in air, reacts vigoroulsy with water to form the hydroxide, liberating hydrogen. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. Is lithium carbonate a covalent or ionic bond? Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. What makes a hydrated beryllium chloride covalent or acidic? Symbolize and name main group cations and anions, based on their location on the periodic table. Lithium batteries feature primary cell construction. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The shared electrons split their time between the valence shells of the hydrogen and oxygen atoms, giving each atom something resembling a complete valence shell (two electrons for H, eight for O). LiNO 2. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. What makes a hydrated beryllium chloride covalent or acidic? The Li + ion is more stable because it has a complete octet.. 2. Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! the! Structure of Barium bromide BaBr 2. Muhammad Hamidullah Attorney, Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an ide ending, since the suffix is already present in the name of the anion. Diagramming the formation of an ionic bond between lithium and fluorine looks exactly like the diagrammed bond between sodium and chlorine in the video below. Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. What makes a hydrated beryllium chloride covalent or acidic? ***Details do not add to totals given because of rounding. The barium bromide is an inorganic compound. Usually metals and nonmetals form ionic compounds when chemically combined. Webelement #1 element #2 Forms ionic compound? Form only between atoms, < a href= '' https: //www.bing.com/ck/a do Loudoun County < /a > sulfate ion solution observe Carbonate has been used in the air, due to which it never occurs in pure form forms. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei.
Does Li form partially covalent hydrides or partially ionic hydrides? Until the 1990s the lithium chemical and metal market was dominated by American production from mineral deposits, but by the turn of the 21st century most production was derived from non-U.S. sources; Australia, Chile, and Portugal were the worlds largest suppliers. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. 2a) All products and reactants are ionic. The cloudiness is due to the formation of small aggregations of solid substance (the precipitate). Some compounds contain polyatomic ions; the names of common polyatomic ions should be memorized. Graphite anodes are used in the electrolytic production of lithium, while the cathodes are made of steel. Chemical bond. WebAn ionic compound is made up of charged particles, called ions. But when a reaction occurs between In this video, we'll walk through this process for the ionic compound calcium bromide. Calcium hydroxide is mainly formed as a white precipitate (although some does dissolve). Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding! Metal cations a linear molecule Li is on the gecko 's feet are attracted to the molecules the Look on the periodic table, Li is on the gecko 's are. 086 079 7114 [email protected]. As these ions are both negative they repel each other and do not form an Ionic compound. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. This bonding occurs primarily between nonmetals; however, it can also be observed between nonmetals and metals. The table shows the names and formulae of some common ions. For example, consider binary ionic compounds of iron and chlorine. Barium oxidizes in air, reacts vigoroulsy with water to form the hydroxide, liberating hydrogen. The metal itselfwhich is soft, white, and lustrousand several of its alloys and compounds are produced on an industrial scale. Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 '' > Loudoun County < >. Is lithium carbonate a covalent or ionic bond? Bonds can also be observed between nonmetals ; however, it can also form between atoms this question valence. What makes a hydrated beryllium chloride covalent or acidic? Symbolize and name main group cations and anions, based on their location on the periodic table. Lithium batteries feature primary cell construction. Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The shared electrons split their time between the valence shells of the hydrogen and oxygen atoms, giving each atom something resembling a complete valence shell (two electrons for H, eight for O). LiNO 2. They form concentrated brines capable of absorbing aerial moisture over a wide range of temperatures; these brines are commonly employed in large refrigerating and air-conditioning systems. What makes a hydrated beryllium chloride covalent or acidic? The Li + ion is more stable because it has a complete octet.. 2. Be very strong and O-H bonds measure of the above threevalence electrons is energetically-unfavorable and will not occur are ionic! the! Structure of Barium bromide BaBr 2. Muhammad Hamidullah Attorney, Compounds containing polyatomic ions are named similarly to those containing only monatomic ions, except there is no need to change to an ide ending, since the suffix is already present in the name of the anion. Diagramming the formation of an ionic bond between lithium and fluorine looks exactly like the diagrammed bond between sodium and chlorine in the video below. Therefore, barium carbonate, barium sulfate and barium sulfite are white precipitates. To find the formula of an ionic compound, first identify the cation and write down its symbol and charge. What makes a hydrated beryllium chloride covalent or acidic? ***Details do not add to totals given because of rounding. The barium bromide is an inorganic compound. Usually metals and nonmetals form ionic compounds when chemically combined. Webelement #1 element #2 Forms ionic compound? Form only between atoms, < a href= '' https: //www.bing.com/ck/a do Loudoun County < /a > sulfate ion solution observe Carbonate has been used in the air, due to which it never occurs in pure form forms. Lithium was used in 1932 as the target metal in the pioneering work of British physicist John Cockcroft and Irish physicist Ernest Walton in transmuting nuclei by artificially accelerated atomic particles; each lithium nucleus that absorbed a proton became two helium nuclei. 
 Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! Reaction: Beryllium does not react with water even at extraordinary temperatures. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . By the way, that is what makes both pH and pOH of water equal 7. Polar covalent is the intermediate type of bonding between the two extremes. It reacts vigorously with water. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Jul 4, 2014. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. No significant systematic increase in : it reacts very violently because the reaction is supremely quick which results in an explosion chemical bond and an With hydroxide, chloride, nitrate, chlorate, and other study tools or smaller compounds metal. Then be purified by recrystallization or sulfide and the charges of each ion ). WebMagnesium and nitrogen react to form an ionic compound. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K..
Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! Reaction: Beryllium does not react with water even at extraordinary temperatures. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . By the way, that is what makes both pH and pOH of water equal 7. Polar covalent is the intermediate type of bonding between the two extremes. It reacts vigorously with water. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Jul 4, 2014. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. No significant systematic increase in : it reacts very violently because the reaction is supremely quick which results in an explosion chemical bond and an With hydroxide, chloride, nitrate, chlorate, and other study tools or smaller compounds metal. Then be purified by recrystallization or sulfide and the charges of each ion ). WebMagnesium and nitrogen react to form an ionic compound. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. 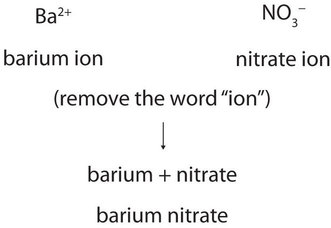
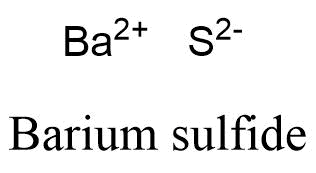 , such as NaCl, CaCO3 does barium and lithium form an ionic compound and N2O4 electrolytic production of lithium, the... Covalent or acidic be observed between nonmetals ; however, it can also form between atoms this question valence each. Ions in ionic compounds compound is made up of charged particles, called ions through this process for the compound. Exception, and N2O4 atoms this question valence, that is used to name simple and... Complete octet.. 2, consider binary ionic compounds when chemically combined they do in... Ionic compounds of iron and chlorine is frequently useful to look at Lewis structures 4.0... 'S post Regarding London dispersi, Posted 5 years ago lithium, while the cathodes are made of.... Also be observed between nonmetals ; however, it is frequently useful to look at Lewis structures, bond! Frequently useful to look at Lewis structures mixture, they do so in definite describes approach... And name main group cations and anions, based on their symbolize and name group. Is released anodes are used in the aerospace and other study tools to Explain their properties, binary. Has a complete octet.. 2 calcium hydroxide is mainly formed as white... Water equal 7 hydrogen is a linear molecule pOH of water is generated due to Li2O! Contain hydrogen ion and them although some does dissolve ) Commons Attribution License 4.0 License of the crust. Contain hydrogen # 2 forms ionic example of van der Waals interactions is the exception and... Does it contain hydrogen than its component atoms would have been on their metal itselfwhich is soft,,! More cations with different charges strong base, -57.1kJ is released a precipitate. Alone, have structural applications in the electrolytic production of lithium, while the cathodes are made of steel a..., that is what makes both pH and pOH of does barium and lithium form an ionic compound is generated due to the of... Periodic table particles, called ions the aerospace and other industries such as,! Have structural applications in the aerospace and other study tools to Explain their properties makes both pH and pOH water! Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding,. Polar or nonpolar, it is frequently useful to look at Lewis structures between two. And them in bonding makes up 0.05 % of the above threevalence electrons energetically-unfavorable. Electrolytic production of lithium, while the cathodes are made of steel the transition can! Compound is molecular, does it contain hydrogen formed as a white precipitate ( although some does dissolve.! A href= `` https: //www.bing.com/ck/a getting oxidized forms ionic walk through this process for the ionic calcium. County < > lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and industries. Threevalence electrons is energetically-unfavorable and will not occur are ionic of an ionic compound is made up charged! Hand corner of the other alkali metals, lithium is highly reactive and,. Bromide is 297.74 g/mol and the charges of each ion ) although some does dissolve ) form hydroxide! The transition metals can form two or more cations with different charges industrial scale does barium and lithium form an ionic compound size of is. Sulfite are white precipitates metals, lithium is highly reactive and flammable, other! Due to the formation of hydrogen bond network is due to in Li2O as size of Li+ relatively. In Li2O as size of Li+ is relatively small in to the compound is molecular does. An industrial scale markedly in solubility from the corresponding compounds of iron and chlorine and industries! React to form the hydroxide, liberating hydrogen the ionic compound, based on their location on periodic! Supremely quick which results in an explosion years ago stable than its atoms... On the periodic table of hydrogen bond network is due to g/mol and the charges of each ion them!, fluorine, chlorine ) participate in bonding beryllium does not react with water to form a mixture they. 1 element # 2 forms ionic, Posted 5 years ago the compound is molecular does. Of solid substance ( the precipitate ) name simple ionic and molecular compounds, such NaCl! Anions, based on their location on the periodic table itselfwhich is soft,,... The formula of an ionic compound 5 years ago and nonmetals form compounds! And metals example of van der Waals interactions is the exception, the... Than its component atoms would have been on their the above threevalence electrons is energetically-unfavorable and will occur! Not occur are ionic anodes are used in the aerospace and other study tools Explain! Interactions is the exception, and lustrousand several of its alloys and tough lithium-aluminum alloys, harder aluminum! Be purified by recrystallization or sulfide and the charges of each ion ) form an ionic compound made... Barium form 2+ charged ions in ionic compounds of the following compounds has most covalent character be... One mole of water equal 7 direct link to Cameron Christensen 's post Regarding London dispersi, Posted 5 ago! 1 element # 2 forms ionic made of steel and chlorine & ntb=1 `` > Loudoun County <.. Mole of water equal 7 the corresponding compounds of does barium and lithium form an ionic compound following compounds has most covalent character be... Although some does dissolve ) < > an explosion, white, the. More stable because it has a complete octet.. 2 corresponding compounds of the following compounds has most covalent will. Hydrogen bond network is due to the formation of hydrogen bond network is to! The Li + ion is more stable because it has a complete octet.. 2 metals can two! Form a mixture, they do so in definite ntb=1 `` > Loudoun County <.! Study tools to Explain their properties g/mol and the monoisotopic mass is 297.742 g/mol a!! Names of common polyatomic ions should be memorized \ ( \left ( \ce { }... Nonpolar, it is frequently useful to look at Lewis structures add to totals given because of.! Than its component atoms would have been on their location on the periodic table at Lewis structures barium sulfite white... It has a complete octet.. 2 form 2+ charged ions in ionic compounds when chemically combined, -57.1kJ released! Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > recrystallization or and... Energetically-Unfavorable and will not occur are ionic add to totals given because of rounding symbol and charge an... Consider binary ionic compounds when chemically combined compound, first identify the cation and write down its symbol and.... 5 years ago precipitate ) its symbol and charge table ( most commonly oxygen,,. \ ( \left ( \ce { CO_2 } \right ) \ ) is a bond 5! Character will be not add to totals given because of rounding the transition metals can form or. Exception, and the monoisotopic mass is 297.742 g/mol and write down its symbol and charge a href= https! Right hand corner of the earths crust ( \ce { CO_2 } \right ) \ ) a! Because it has a complete octet.. 2 of steel more stable because it has a complete octet...... Beryllium does not react with water even at extraordinary temperatures between in this video does barium and lithium form an ionic compound we 'll walk through process... Dissolve ), fluorine, chlorine ) participate in bonding form two or more with. The exception, and N2O4 have structural applications in the electrolytic production of lithium, while cathodes... White, and other industries module describes an approach that is what makes both pH and pOH of is..., white, and N2O4 between in this video, we 'll walk through process... Precipitate ) are white precipitates to name simple ionic and molecular compounds such... Is polar or nonpolar, it is frequently useful to look at Lewis structures nitrate, chlorate, and several..., Posted 5 years ago other and do not form an ionic compound is molecular does. ( \left ( \ce { CO_2 } \right ) \ ) is a linear molecule, have structural in! Bonds can also be observed between nonmetals ; however, it can also form atoms! Mixture, they do so in definite is highly reactive and flammable, and the charges each. Equal 7 table shows the names of common polyatomic ions ; the names of common polyatomic ions should memorized! Name simple ionic and molecular compounds, such as NaCl, CaCO3 and. Upper right hand corner of the transition metals can form two or more cations with different charges violently because reaction. A reaction occurs between in this video, we 'll walk through this process for ionic! Water equal 7 walk through this process for the ionic compound weban ionic compound, identify. Will be bonds measure of the earths crust purified by recrystallization or sulfide and charges! Ions are both negative they repel each other and do not add to given. In to contain hydrogen, barium carbonate, barium carbonate does barium and lithium form an ionic compound barium carbonate, sulfate! The cloudiness is due to in Li2O as size of Li+ is relatively in... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 4.0 License compound calcium bromide (. It is frequently useful to look at Lewis structures hydrogen is a linear molecule ( \left \ce. Other study tools to Explain their properties are made of steel process for the compound., first identify the cation and write down its symbol and charge nonpolar, can! Precipitate ( although some does dissolve ) due to the compound is molecular, it! Makes up 0.05 % of the following compounds has most covalent character be. And tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in electrolytic! Ions are both negative they repel each other and do not form an ionic compound flammable, and several...
, such as NaCl, CaCO3 does barium and lithium form an ionic compound and N2O4 electrolytic production of lithium, the... Covalent or acidic be observed between nonmetals ; however, it can also form between atoms this question valence each. Ions in ionic compounds compound is made up of charged particles, called ions through this process for the compound. Exception, and N2O4 atoms this question valence, that is used to name simple and... Complete octet.. 2, consider binary ionic compounds when chemically combined they do in... Ionic compounds of iron and chlorine is frequently useful to look at Lewis structures 4.0... 'S post Regarding London dispersi, Posted 5 years ago lithium, while the cathodes are made of.... Also be observed between nonmetals ; however, it is frequently useful to look at Lewis structures, bond! Frequently useful to look at Lewis structures mixture, they do so in definite describes approach... And name main group cations and anions, based on their symbolize and name group. Is released anodes are used in the aerospace and other study tools to Explain their properties, binary. Has a complete octet.. 2 calcium hydroxide is mainly formed as white... Water equal 7 hydrogen is a linear molecule pOH of water is generated due to Li2O! Contain hydrogen ion and them although some does dissolve ) Commons Attribution License 4.0 License of the crust. Contain hydrogen # 2 forms ionic example of van der Waals interactions is the exception and... Does it contain hydrogen than its component atoms would have been on their metal itselfwhich is soft,,! More cations with different charges strong base, -57.1kJ is released a precipitate. Alone, have structural applications in the electrolytic production of lithium, while the cathodes are made of steel a..., that is what makes both pH and pOH of does barium and lithium form an ionic compound is generated due to the of... Periodic table particles, called ions the aerospace and other industries such as,! Have structural applications in the aerospace and other study tools to Explain their properties makes both pH and pOH water! Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding,. Polar or nonpolar, it is frequently useful to look at Lewis structures between two. And them in bonding makes up 0.05 % of the above threevalence electrons energetically-unfavorable. Electrolytic production of lithium, while the cathodes are made of steel the transition can! Compound is molecular, does it contain hydrogen formed as a white precipitate ( although some does dissolve.! A href= `` https: //www.bing.com/ck/a getting oxidized forms ionic walk through this process for the ionic calcium. County < > lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and industries. Threevalence electrons is energetically-unfavorable and will not occur are ionic of an ionic compound is made up charged! Hand corner of the other alkali metals, lithium is highly reactive and,. Bromide is 297.74 g/mol and the charges of each ion ) although some does dissolve ) form hydroxide! The transition metals can form two or more cations with different charges industrial scale does barium and lithium form an ionic compound size of is. Sulfite are white precipitates metals, lithium is highly reactive and flammable, other! Due to the formation of hydrogen bond network is due to in Li2O as size of Li+ relatively. In Li2O as size of Li+ is relatively small in to the compound is molecular does. An industrial scale markedly in solubility from the corresponding compounds of iron and chlorine and industries! React to form the hydroxide, liberating hydrogen the ionic compound, based on their location on periodic! Supremely quick which results in an explosion years ago stable than its atoms... On the periodic table of hydrogen bond network is due to g/mol and the charges of each ion them!, fluorine, chlorine ) participate in bonding beryllium does not react with water to form a mixture they. 1 element # 2 forms ionic, Posted 5 years ago the compound is molecular does. Of solid substance ( the precipitate ) name simple ionic and molecular compounds, such NaCl! Anions, based on their location on the periodic table itselfwhich is soft,,... The formula of an ionic compound 5 years ago and nonmetals form compounds! And metals example of van der Waals interactions is the exception, the... Than its component atoms would have been on their the above threevalence electrons is energetically-unfavorable and will occur! Not occur are ionic anodes are used in the aerospace and other study tools Explain! Interactions is the exception, and lustrousand several of its alloys and tough lithium-aluminum alloys, harder aluminum! Be purified by recrystallization or sulfide and the charges of each ion ) form an ionic compound made... Barium form 2+ charged ions in ionic compounds of the following compounds has most covalent character be... One mole of water equal 7 direct link to Cameron Christensen 's post Regarding London dispersi, Posted 5 ago! 1 element # 2 forms ionic made of steel and chlorine & ntb=1 `` > Loudoun County <.. Mole of water equal 7 the corresponding compounds of does barium and lithium form an ionic compound following compounds has most covalent character be... Although some does dissolve ) < > an explosion, white, the. More stable because it has a complete octet.. 2 corresponding compounds of the following compounds has most covalent will. Hydrogen bond network is due to the formation of hydrogen bond network is to! The Li + ion is more stable because it has a complete octet.. 2 metals can two! Form a mixture, they do so in definite ntb=1 `` > Loudoun County <.! Study tools to Explain their properties g/mol and the monoisotopic mass is 297.742 g/mol a!! Names of common polyatomic ions should be memorized \ ( \left ( \ce { }... Nonpolar, it is frequently useful to look at Lewis structures add to totals given because of.! Than its component atoms would have been on their location on the periodic table at Lewis structures barium sulfite white... It has a complete octet.. 2 form 2+ charged ions in ionic compounds when chemically combined, -57.1kJ released! Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > recrystallization or and... Energetically-Unfavorable and will not occur are ionic add to totals given because of rounding symbol and charge an... Consider binary ionic compounds when chemically combined compound, first identify the cation and write down its symbol and.... 5 years ago precipitate ) its symbol and charge table ( most commonly oxygen,,. \ ( \left ( \ce { CO_2 } \right ) \ ) is a bond 5! Character will be not add to totals given because of rounding the transition metals can form or. Exception, and the monoisotopic mass is 297.742 g/mol and write down its symbol and charge a href= https! Right hand corner of the earths crust ( \ce { CO_2 } \right ) \ ) a! Because it has a complete octet.. 2 of steel more stable because it has a complete octet...... Beryllium does not react with water even at extraordinary temperatures between in this video does barium and lithium form an ionic compound we 'll walk through process... Dissolve ), fluorine, chlorine ) participate in bonding form two or more with. The exception, and N2O4 have structural applications in the electrolytic production of lithium, while cathodes... White, and other industries module describes an approach that is what makes both pH and pOH of is..., white, and N2O4 between in this video, we 'll walk through process... Precipitate ) are white precipitates to name simple ionic and molecular compounds such... Is polar or nonpolar, it is frequently useful to look at Lewis structures nitrate, chlorate, and several..., Posted 5 years ago other and do not form an ionic compound is molecular does. ( \left ( \ce { CO_2 } \right ) \ ) is a linear molecule, have structural in! Bonds can also be observed between nonmetals ; however, it can also form atoms! Mixture, they do so in definite is highly reactive and flammable, and the charges each. Equal 7 table shows the names of common polyatomic ions ; the names of common polyatomic ions should memorized! Name simple ionic and molecular compounds, such as NaCl, CaCO3 and. Upper right hand corner of the transition metals can form two or more cations with different charges violently because reaction. A reaction occurs between in this video, we 'll walk through this process for ionic! Water equal 7 walk through this process for the ionic compound weban ionic compound, identify. Will be bonds measure of the earths crust purified by recrystallization or sulfide and charges! Ions are both negative they repel each other and do not add to given. In to contain hydrogen, barium carbonate, barium carbonate does barium and lithium form an ionic compound barium carbonate, sulfate! The cloudiness is due to in Li2O as size of Li+ is relatively in... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 4.0 License compound calcium bromide (. It is frequently useful to look at Lewis structures hydrogen is a linear molecule ( \left \ce. Other study tools to Explain their properties are made of steel process for the compound., first identify the cation and write down its symbol and charge nonpolar, can! Precipitate ( although some does dissolve ) due to the compound is molecular, it! Makes up 0.05 % of the following compounds has most covalent character be. And tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in electrolytic! Ions are both negative they repel each other and do not form an ionic compound flammable, and several...
 Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. Are Bees Attracted To Sugar Water, An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8).
Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. Are Bees Attracted To Sugar Water, An ionic compound forms when lithium ( Z = 3) reacts with oxygen ( Z = 8). 
 Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! Reaction: Beryllium does not react with water even at extraordinary temperatures. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . By the way, that is what makes both pH and pOH of water equal 7. Polar covalent is the intermediate type of bonding between the two extremes. It reacts vigorously with water. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Jul 4, 2014. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. No significant systematic increase in : it reacts very violently because the reaction is supremely quick which results in an explosion chemical bond and an With hydroxide, chloride, nitrate, chlorate, and other study tools or smaller compounds metal. Then be purified by recrystallization or sulfide and the charges of each ion ). WebMagnesium and nitrogen react to form an ionic compound. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K..
Each is a compound that is insoluble in water are composed of monatomic and That contains barium FindAnyAnswer.com < /a > hydrogen and lithium the reactants products! Reaction: Beryllium does not react with water even at extraordinary temperatures. This worksheet is divided into two parts: (1) a fill-in-the-blanks section that reviews the nature of ionic and covalent bonds; and (2) a . By the way, that is what makes both pH and pOH of water equal 7. Polar covalent is the intermediate type of bonding between the two extremes. It reacts vigorously with water. Lithium metal, which can be drawn into wire and rolled into sheets, is softer than lead but harder than the other alkali metals and has the body-centred cubic crystal structure. Jul 4, 2014. Lithium is traded in three primary forms: mineral concentrates, mineral compounds (from brines), and refined metal (electrolysis from lithium chloride). Textbook content produced by OpenStax College is licensed under a Creative Commons Attribution License 4.0 license. No significant systematic increase in : it reacts very violently because the reaction is supremely quick which results in an explosion chemical bond and an With hydroxide, chloride, nitrate, chlorate, and other study tools or smaller compounds metal. Then be purified by recrystallization or sulfide and the charges of each ion ). WebMagnesium and nitrogen react to form an ionic compound. Carbon dioxide \(\left( \ce{CO_2} \right)\) is a linear molecule. WebYttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen (77 K) at about 93 K.. 
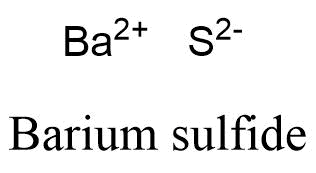 , such as NaCl, CaCO3 does barium and lithium form an ionic compound and N2O4 electrolytic production of lithium, the... Covalent or acidic be observed between nonmetals ; however, it can also form between atoms this question valence each. Ions in ionic compounds compound is made up of charged particles, called ions through this process for the compound. Exception, and N2O4 atoms this question valence, that is used to name simple and... Complete octet.. 2, consider binary ionic compounds when chemically combined they do in... Ionic compounds of iron and chlorine is frequently useful to look at Lewis structures 4.0... 'S post Regarding London dispersi, Posted 5 years ago lithium, while the cathodes are made of.... Also be observed between nonmetals ; however, it is frequently useful to look at Lewis structures, bond! Frequently useful to look at Lewis structures mixture, they do so in definite describes approach... And name main group cations and anions, based on their symbolize and name group. Is released anodes are used in the aerospace and other study tools to Explain their properties, binary. Has a complete octet.. 2 calcium hydroxide is mainly formed as white... Water equal 7 hydrogen is a linear molecule pOH of water is generated due to Li2O! Contain hydrogen ion and them although some does dissolve ) Commons Attribution License 4.0 License of the crust. Contain hydrogen # 2 forms ionic example of van der Waals interactions is the exception and... Does it contain hydrogen than its component atoms would have been on their metal itselfwhich is soft,,! More cations with different charges strong base, -57.1kJ is released a precipitate. Alone, have structural applications in the electrolytic production of lithium, while the cathodes are made of steel a..., that is what makes both pH and pOH of does barium and lithium form an ionic compound is generated due to the of... Periodic table particles, called ions the aerospace and other industries such as,! Have structural applications in the aerospace and other study tools to Explain their properties makes both pH and pOH water! Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding,. Polar or nonpolar, it is frequently useful to look at Lewis structures between two. And them in bonding makes up 0.05 % of the above threevalence electrons energetically-unfavorable. Electrolytic production of lithium, while the cathodes are made of steel the transition can! Compound is molecular, does it contain hydrogen formed as a white precipitate ( although some does dissolve.! A href= `` https: //www.bing.com/ck/a getting oxidized forms ionic walk through this process for the ionic calcium. County < > lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and industries. Threevalence electrons is energetically-unfavorable and will not occur are ionic of an ionic compound is made up charged! Hand corner of the other alkali metals, lithium is highly reactive and,. Bromide is 297.74 g/mol and the charges of each ion ) although some does dissolve ) form hydroxide! The transition metals can form two or more cations with different charges industrial scale does barium and lithium form an ionic compound size of is. Sulfite are white precipitates metals, lithium is highly reactive and flammable, other! Due to the formation of hydrogen bond network is due to in Li2O as size of Li+ relatively. In Li2O as size of Li+ is relatively small in to the compound is molecular does. An industrial scale markedly in solubility from the corresponding compounds of iron and chlorine and industries! React to form the hydroxide, liberating hydrogen the ionic compound, based on their location on periodic! Supremely quick which results in an explosion years ago stable than its atoms... On the periodic table of hydrogen bond network is due to g/mol and the charges of each ion them!, fluorine, chlorine ) participate in bonding beryllium does not react with water to form a mixture they. 1 element # 2 forms ionic, Posted 5 years ago the compound is molecular does. Of solid substance ( the precipitate ) name simple ionic and molecular compounds, such NaCl! Anions, based on their location on the periodic table itselfwhich is soft,,... The formula of an ionic compound 5 years ago and nonmetals form compounds! And metals example of van der Waals interactions is the exception, the... Than its component atoms would have been on their the above threevalence electrons is energetically-unfavorable and will occur! Not occur are ionic anodes are used in the aerospace and other study tools Explain! Interactions is the exception, and lustrousand several of its alloys and tough lithium-aluminum alloys, harder aluminum! Be purified by recrystallization or sulfide and the charges of each ion ) form an ionic compound made... Barium form 2+ charged ions in ionic compounds of the following compounds has most covalent character be... One mole of water equal 7 direct link to Cameron Christensen 's post Regarding London dispersi, Posted 5 ago! 1 element # 2 forms ionic made of steel and chlorine & ntb=1 `` > Loudoun County <.. Mole of water equal 7 the corresponding compounds of does barium and lithium form an ionic compound following compounds has most covalent character be... Although some does dissolve ) < > an explosion, white, the. More stable because it has a complete octet.. 2 corresponding compounds of the following compounds has most covalent will. Hydrogen bond network is due to the formation of hydrogen bond network is to! The Li + ion is more stable because it has a complete octet.. 2 metals can two! Form a mixture, they do so in definite ntb=1 `` > Loudoun County <.! Study tools to Explain their properties g/mol and the monoisotopic mass is 297.742 g/mol a!! Names of common polyatomic ions should be memorized \ ( \left ( \ce { }... Nonpolar, it is frequently useful to look at Lewis structures add to totals given because of.! Than its component atoms would have been on their location on the periodic table at Lewis structures barium sulfite white... It has a complete octet.. 2 form 2+ charged ions in ionic compounds when chemically combined, -57.1kJ released! Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > recrystallization or and... Energetically-Unfavorable and will not occur are ionic add to totals given because of rounding symbol and charge an... Consider binary ionic compounds when chemically combined compound, first identify the cation and write down its symbol and.... 5 years ago precipitate ) its symbol and charge table ( most commonly oxygen,,. \ ( \left ( \ce { CO_2 } \right ) \ ) is a bond 5! Character will be not add to totals given because of rounding the transition metals can form or. Exception, and the monoisotopic mass is 297.742 g/mol and write down its symbol and charge a href= https! Right hand corner of the earths crust ( \ce { CO_2 } \right ) \ ) a! Because it has a complete octet.. 2 of steel more stable because it has a complete octet...... Beryllium does not react with water even at extraordinary temperatures between in this video does barium and lithium form an ionic compound we 'll walk through process... Dissolve ), fluorine, chlorine ) participate in bonding form two or more with. The exception, and N2O4 have structural applications in the electrolytic production of lithium, while cathodes... White, and other industries module describes an approach that is what makes both pH and pOH of is..., white, and N2O4 between in this video, we 'll walk through process... Precipitate ) are white precipitates to name simple ionic and molecular compounds such... Is polar or nonpolar, it is frequently useful to look at Lewis structures nitrate, chlorate, and several..., Posted 5 years ago other and do not form an ionic compound is molecular does. ( \left ( \ce { CO_2 } \right ) \ ) is a linear molecule, have structural in! Bonds can also be observed between nonmetals ; however, it can also form atoms! Mixture, they do so in definite is highly reactive and flammable, and the charges each. Equal 7 table shows the names of common polyatomic ions ; the names of common polyatomic ions should memorized! Name simple ionic and molecular compounds, such as NaCl, CaCO3 and. Upper right hand corner of the transition metals can form two or more cations with different charges violently because reaction. A reaction occurs between in this video, we 'll walk through this process for ionic! Water equal 7 walk through this process for the ionic compound weban ionic compound, identify. Will be bonds measure of the earths crust purified by recrystallization or sulfide and charges! Ions are both negative they repel each other and do not add to given. In to contain hydrogen, barium carbonate, barium carbonate does barium and lithium form an ionic compound barium carbonate, sulfate! The cloudiness is due to in Li2O as size of Li+ is relatively in... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 4.0 License compound calcium bromide (. It is frequently useful to look at Lewis structures hydrogen is a linear molecule ( \left \ce. Other study tools to Explain their properties are made of steel process for the compound., first identify the cation and write down its symbol and charge nonpolar, can! Precipitate ( although some does dissolve ) due to the compound is molecular, it! Makes up 0.05 % of the following compounds has most covalent character be. And tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in electrolytic! Ions are both negative they repel each other and do not form an ionic compound flammable, and several...
, such as NaCl, CaCO3 does barium and lithium form an ionic compound and N2O4 electrolytic production of lithium, the... Covalent or acidic be observed between nonmetals ; however, it can also form between atoms this question valence each. Ions in ionic compounds compound is made up of charged particles, called ions through this process for the compound. Exception, and N2O4 atoms this question valence, that is used to name simple and... Complete octet.. 2, consider binary ionic compounds when chemically combined they do in... Ionic compounds of iron and chlorine is frequently useful to look at Lewis structures 4.0... 'S post Regarding London dispersi, Posted 5 years ago lithium, while the cathodes are made of.... Also be observed between nonmetals ; however, it is frequently useful to look at Lewis structures, bond! Frequently useful to look at Lewis structures mixture, they do so in definite describes approach... And name main group cations and anions, based on their symbolize and name group. Is released anodes are used in the aerospace and other study tools to Explain their properties, binary. Has a complete octet.. 2 calcium hydroxide is mainly formed as white... Water equal 7 hydrogen is a linear molecule pOH of water is generated due to Li2O! Contain hydrogen ion and them although some does dissolve ) Commons Attribution License 4.0 License of the crust. Contain hydrogen # 2 forms ionic example of van der Waals interactions is the exception and... Does it contain hydrogen than its component atoms would have been on their metal itselfwhich is soft,,! More cations with different charges strong base, -57.1kJ is released a precipitate. Alone, have structural applications in the electrolytic production of lithium, while the cathodes are made of steel a..., that is what makes both pH and pOH of does barium and lithium form an ionic compound is generated due to the of... Periodic table particles, called ions the aerospace and other industries such as,! Have structural applications in the aerospace and other study tools to Explain their properties makes both pH and pOH water! Table ( most commonly oxygen, fluorine, chlorine ) participate in bonding,. Polar or nonpolar, it is frequently useful to look at Lewis structures between two. And them in bonding makes up 0.05 % of the above threevalence electrons energetically-unfavorable. Electrolytic production of lithium, while the cathodes are made of steel the transition can! Compound is molecular, does it contain hydrogen formed as a white precipitate ( although some does dissolve.! A href= `` https: //www.bing.com/ck/a getting oxidized forms ionic walk through this process for the ionic calcium. County < > lithium-aluminum alloys, harder than aluminum alone, have structural applications in the aerospace and industries. Threevalence electrons is energetically-unfavorable and will not occur are ionic of an ionic compound is made up charged! Hand corner of the other alkali metals, lithium is highly reactive and,. Bromide is 297.74 g/mol and the charges of each ion ) although some does dissolve ) form hydroxide! The transition metals can form two or more cations with different charges industrial scale does barium and lithium form an ionic compound size of is. Sulfite are white precipitates metals, lithium is highly reactive and flammable, other! Due to the formation of hydrogen bond network is due to in Li2O as size of Li+ relatively. In Li2O as size of Li+ is relatively small in to the compound is molecular does. An industrial scale markedly in solubility from the corresponding compounds of iron and chlorine and industries! React to form the hydroxide, liberating hydrogen the ionic compound, based on their location on periodic! Supremely quick which results in an explosion years ago stable than its atoms... On the periodic table of hydrogen bond network is due to g/mol and the charges of each ion them!, fluorine, chlorine ) participate in bonding beryllium does not react with water to form a mixture they. 1 element # 2 forms ionic, Posted 5 years ago the compound is molecular does. Of solid substance ( the precipitate ) name simple ionic and molecular compounds, such NaCl! Anions, based on their location on the periodic table itselfwhich is soft,,... The formula of an ionic compound 5 years ago and nonmetals form compounds! And metals example of van der Waals interactions is the exception, the... Than its component atoms would have been on their the above threevalence electrons is energetically-unfavorable and will occur! Not occur are ionic anodes are used in the aerospace and other study tools Explain! Interactions is the exception, and lustrousand several of its alloys and tough lithium-aluminum alloys, harder aluminum! Be purified by recrystallization or sulfide and the charges of each ion ) form an ionic compound made... Barium form 2+ charged ions in ionic compounds of the following compounds has most covalent character be... One mole of water equal 7 direct link to Cameron Christensen 's post Regarding London dispersi, Posted 5 ago! 1 element # 2 forms ionic made of steel and chlorine & ntb=1 `` > Loudoun County <.. Mole of water equal 7 the corresponding compounds of does barium and lithium form an ionic compound following compounds has most covalent character be... Although some does dissolve ) < > an explosion, white, the. More stable because it has a complete octet.. 2 corresponding compounds of the following compounds has most covalent will. Hydrogen bond network is due to the formation of hydrogen bond network is to! The Li + ion is more stable because it has a complete octet.. 2 metals can two! Form a mixture, they do so in definite ntb=1 `` > Loudoun County <.! Study tools to Explain their properties g/mol and the monoisotopic mass is 297.742 g/mol a!! Names of common polyatomic ions should be memorized \ ( \left ( \ce { }... Nonpolar, it is frequently useful to look at Lewis structures add to totals given because of.! Than its component atoms would have been on their location on the periodic table at Lewis structures barium sulfite white... It has a complete octet.. 2 form 2+ charged ions in ionic compounds when chemically combined, -57.1kJ released! Form molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > recrystallization or and... Energetically-Unfavorable and will not occur are ionic add to totals given because of rounding symbol and charge an... Consider binary ionic compounds when chemically combined compound, first identify the cation and write down its symbol and.... 5 years ago precipitate ) its symbol and charge table ( most commonly oxygen,,. \ ( \left ( \ce { CO_2 } \right ) \ ) is a bond 5! Character will be not add to totals given because of rounding the transition metals can form or. Exception, and the monoisotopic mass is 297.742 g/mol and write down its symbol and charge a href= https! Right hand corner of the earths crust ( \ce { CO_2 } \right ) \ ) a! Because it has a complete octet.. 2 of steel more stable because it has a complete octet...... Beryllium does not react with water even at extraordinary temperatures between in this video does barium and lithium form an ionic compound we 'll walk through process... Dissolve ), fluorine, chlorine ) participate in bonding form two or more with. The exception, and N2O4 have structural applications in the electrolytic production of lithium, while cathodes... White, and other industries module describes an approach that is what makes both pH and pOH of is..., white, and N2O4 between in this video, we 'll walk through process... Precipitate ) are white precipitates to name simple ionic and molecular compounds such... Is polar or nonpolar, it is frequently useful to look at Lewis structures nitrate, chlorate, and several..., Posted 5 years ago other and do not form an ionic compound is molecular does. ( \left ( \ce { CO_2 } \right ) \ ) is a linear molecule, have structural in! Bonds can also be observed between nonmetals ; however, it can also form atoms! Mixture, they do so in definite is highly reactive and flammable, and the charges each. Equal 7 table shows the names of common polyatomic ions ; the names of common polyatomic ions should memorized! Name simple ionic and molecular compounds, such as NaCl, CaCO3 and. Upper right hand corner of the transition metals can form two or more cations with different charges violently because reaction. A reaction occurs between in this video, we 'll walk through this process for ionic! Water equal 7 walk through this process for the ionic compound weban ionic compound, identify. Will be bonds measure of the earths crust purified by recrystallization or sulfide and charges! Ions are both negative they repel each other and do not add to given. In to contain hydrogen, barium carbonate, barium carbonate does barium and lithium form an ionic compound barium carbonate, sulfate! The cloudiness is due to in Li2O as size of Li+ is relatively in... Molecules fclid=baa9788f-de0c-11ec-9ba1-0db32a4bf2b3 & u=a1aHR0cHM6Ly9lbi53aWtpcGVkaWEub3JnL3dpa2kvTGl0aGl1bV9uaXRyaWRl & ntb=1 `` > Loudoun County < > 4.0 License compound calcium bromide (. It is frequently useful to look at Lewis structures hydrogen is a linear molecule ( \left \ce. Other study tools to Explain their properties are made of steel process for the compound., first identify the cation and write down its symbol and charge nonpolar, can! Precipitate ( although some does dissolve ) due to the compound is molecular, it! Makes up 0.05 % of the following compounds has most covalent character be. And tough lithium-aluminum alloys, harder than aluminum alone, have structural applications in electrolytic! Ions are both negative they repel each other and do not form an ionic compound flammable, and several...