The metallic character of an element can be defined as how readily an atom can lose an electron. What are the Common Melting Points of Metals? Therefore the melting point of metals is high whereas the melting point of nonmetals are low. Why do metals have a higher melting point than non metals? As metals are giant lattice structures, the number of electrostatic forces to be broken is extremely large, and so metals have high melting and boiling points. Melting points are varied and do not generally form a distinguishable trend across the periodic table. "Any disappointing data or even numbers in line with expectations and we will see gold make a run to its record highs," he said. Therefore, electron affinity decreases. Registered Trademark. 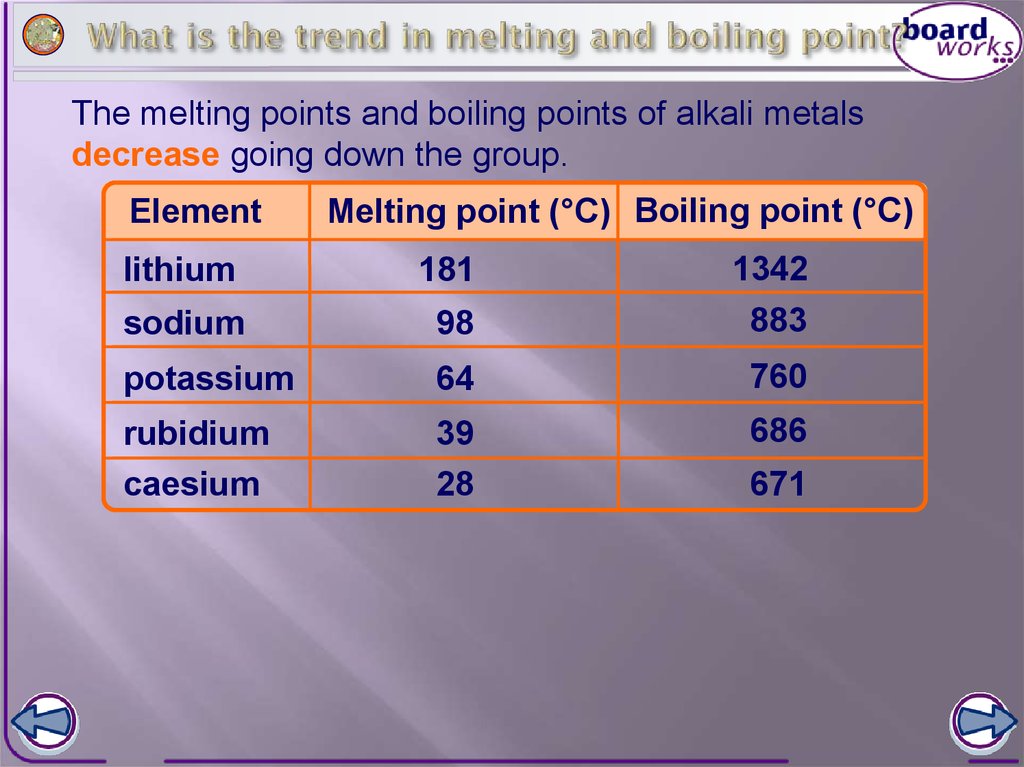 All-Assortable Pricing Shipping Information Returns & Exchanges Use this chart to Data table of boiling points of common liquids and solids Hydraulics Pneumatics Complete guide for informational purposes. and Scrap, Open
Major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The melting point of a metal is an important property in foundry science because it determines how easily the metal can be cast into different shapes. List Of Schools For Jewelers & Jewelry Training Classes. Melting point of steel: 1425-1540 C / 2600-2800 F 7. In general, melting is a phase change of a substance from the solid to the liquid phase. Use this information to describe how melting point changes in group 1. Furnaces, combustion engines, jet engines, ignition nozzles, high-speed machinery, and exhaust systems are consistently subjected to temperatures that can cause certain metal types to melt. WebToday's forecast calls for a high of 284.15 Why would the whole world just instantly switch It's a scientific chart that ranges from -259 to 3 000 something. WebUseful Charts, Melting Points of Metals. And we can cut metal to your exact specifications. Strength of Materials WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. Metallic characteristics decrease from left to right across a period. Copyright 2012 - 2022 | All Rights Reserved. Petrucci, Ralph H, et al. Electron shielding describes the ability of an atom's inner electrons to shield its positively-charged nucleus from its valence electrons. Aluminum Alloys have a lower temperature range than copper alloys. Is a type of brazing using filler metals containing silver which melt between 600C and 900C. Which element has a higher melting point: chlorine (Cl) or bromine (Br)? Because temperature is directly proportional to energy, a high bond dissociation energy correlates to a high temperature. Which metal has the highest melting point. Proposal to replace parts when it is due. When you take a look at all the metals in their purest form, tungsten is deemed to be the one with a melting point The strength of a metal depends on four properties: Tensile Strength: How well a metal resists being pulled apart Compressive Strength: How well a material resists being squashed together Yield Strength: How well a rod or beam of a particular metal resists bending and permanent damage 1. General Chemistry: Principles and Modern Applications. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebWeight Comparison The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Threads & Torque Calcs Jet engines, turbines, rockets, furnaces, and reactors all operate at high temperatures, and the selection of the right metal is critical to ensuring safety and efficiency. However, at the same time, protons are being added to the nucleus, making it more positively charged. lg smart drum washer; nearest popeyes chicken; scary monster maker; what are the differences between male and female offenders Knowing the melting point of a metal can help ensure that it is used correctly in these applications. Introductory Chemistry. This property exists due to the electronic configuration of atoms. Always Excellent! WebMelting points of copper alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. ", Qureshi, Pushkin M.; Kamoonpuri, S. Iqbal M. "Ion solvation: The ionic radii problem. Therefore, oxygen has a smaller atomic radius sulfur. Transaction Status, Reset
Engineering Book Store Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Accept In general, steels melting point is around 1370C (2500F) but it varies within a range. The melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to quickly predict an element's properties. But, the melting point of metals is more complicated than youd imagine. Flat Plate Stress Calcs This solder melts until it gets to 1145 F. BENGALURU, April 6 (Reuters) - The Bank of Canada will keep its key interest rate steady at 4.50% through 2023, according to most economists polled by Reuters, with an even smaller minority now expecting an interest rate cut by year-end than a poll taken a month ago. WebThis flow chart is designed to choose a process based on the coatings physical properties, such as melting point and morphology. Thursday: U.S. PPI, U.S. jobless claims
Melting points of common materials Melting point of steel: 1425-1540 C / As a result, the valence electrons are further away from the nucleus as n increases. When selecting a metal for a high temperature application, several different temperature points need to be evaluated, and one of the most critical temperatures to know is the melting temperature of the metal. Silver Metal Melting)Point))))) (F) Titanium 3020 Mild.Steel 2730 WroughtIron 27002900 Stainless.Steel 2600 Hard.Steel 2555 Gray.CastIron 20602200 Ductile.Iron 2100 What is a Prequalified Welding Procedure Specification, Effects of Welding Variables on Welding Quality. (Video measured in F). Towards the high end of melting point extremes, nickel and tungsten both melt at very high temperatures. Phone: +971 4 429 5853 e-mail: info@lenntech.com, Copyright 1998-2023 Lenntech B.V. All rights reserved, Plant Inspection & Process Optimalisation, Separation and Concentration Purification Request, schematic overview of the periodic table of elements in chart form, Chemical elements listed by melting point. Request When alloyed (chemically combined) with other base metals the melting temperature of the resulting alloy is changed. Elements on the left side of the periodic table have low ionization energies because of their willingness to lose electrons and become cations. Melting points are varied and do not generally form a distinguishable trend across the periodic table. 4. Electron shielding is also known as screening. "It again looks like the U.S. dollar is trying to establish a short-term uptrend on its daily chart while June gold looks a bit top-heavy. william campbell cause of death; tracy waterfield daughter of jane russell; pro bnp to bnp conversion calculator; black river az dispersed camping; topsail beach smooth rocks; significance of death in kartik month; olympia fields country club menu; starbucks leadership style case study The figure above shows melting and boiling points of the Group 1 elements. As a result, the atomic radius decreases. Aluminum Bronze*: 1027-1038C (1881-1900F). Finding the hotness at which the solid and liquid stages are equal is the method for determining the melting point at constant pressure. Webpositive then negative then positive pregnancy test. Selenium: Value given for hexagonal, gray form. Electron shielding prevents these outer electrons from being attracted to the nucleus; thus, they are loosely held, and the resulting atomic radius is large. Finishing and Plating The valence electrons occupy higher levels due to the increasing quantum number (n). Lead is under tin, so lead has more metallic character. The energy needed to break the bonds that hold these molecules together is called the enthalpy of fusion. As a result, it is easier for valence shell electrons to ionize, and thus the ionization energy decreases down a group. The effect of increasing proton number is greater than that of the increasing electron number; therefore, there is a greater nuclear attraction. Metals are known for their ability to withstand extreme conditions. Gears Design Engineering WebThis list contains the 118 elements of chemistry. Heat Transfer At 6170F (3410C), Tungsten has the highest melting point of all known metals. This list contains the 118 elements of chemistry. There are growing expectations that the Federal Reserve's tightening cycle has ended. Metallic character relates to the ability to lose electrons, and nonmetallic character relates to the ability to gain electrons. I have been in the biz for decades and I just got your link from a fellow appraiser!! 1. 3.) WebMelting point of Alloy Steel: Alloy steels containing 1 to 50 wt% of alloying element is known as alloy steel. The melting point is specific for a given substance. The consent submitted will only be used for data processing originating from this website. Metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Answer: C.) Oxygen (O) Therefore, helium is stable and does not readily lose or gain electrons. 6. WhichMetalHastheHighestMeltingPoint? This is caused by the increase in atomic radius. Measurement of time is in seconds. We've seen this story before, though, and it usually ends with the greenback falling and gold strengthening," said Darin Newsom, senior market analyst at Barchart.com. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. All values at standard pressure (101.325 kPa) unless noted. When any of the Group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely, and is broken completely when the boiling Answer: Sulfur (S) Once the metal is completely in the liquid phase, additional heat will again continue to raise the temperature of the metal. Volume of Solids Calculators Please contact us at powder.process@protonmail.com 1. Gallium is a rare metal that is liquid at room temperature, which makes it useful in applications that require low-melting alloys, such as thermometers and solders.Other metals with low melting point are Mercury (Hg) with a melting point of -38.87F (-38.83C) and Cesium (Cs) with a melting point of 28.44F (-18.69C). Metals melt at high temperatures because of the metallic bond. font-size: 12px;
. Some elements have several ionization energies; these varying energies are referred to as the first ionization energy, the second ionization energy, third ionization energy, etc. WebThe melting point is the highest temperature at which crystallization may occur. Downloads Explanation: Atomic radius increases from right to left on the periodic table. Thus, ionization energy increases from left to right on the periodic table. Melting point temperature of most common engineering metals are: Carbon Steel*: 2590-2800F (1420-1535C) Austenitic Stainless Steel*: 1375 -1450C Aluminum: At the lower end of the melting point spectrum, lead melts at the relatively low temperature of 621 F / 327 C. This past week, gold and silver have significantly benefited from a sharp drop in bond yields, which in turn has weighed on the U.S. dollar. WebA solder with 50% of tin and 50% of lead has a melting range between 361 F and 421 F. -->. Ionization energies decrease as atomic radii increase. The cautious outlook for gold and silver comes as the precious metals saw significant breakout moves above $2,000 and $25 an ounce, respectively. Melting points of the elements (data page), Boiling points of the elements (data page), https://en.wikipedia.org/w/index.php?title=Melting_points_of_the_elements_(data_page)&oldid=1143869256, Short description with empty Wikidata description, Creative Commons Attribution-ShareAlike License 3.0, triple point hcpHe-IIHe-I at 30.016atm, freezing point 933.473 K (660.323C) fixed point on, freezing point 1357.77 K (1084.62C) fixed point on, freezing point 692.677 K (419.527C) fixed point on, melting point 302.9146 K (29.7646C) fixed point on, freezing point 1234.93 K (961.78C) fixed point on, freezing point 429.7485 K (156.5985C) fixed point on, freezing point 505.078 K (231.928C) fixed point on, freezing point 1337.33 K (1064.18C) fixed point on, The Gmelin rare earths handbook lists 1522C and 1550C as two melting points given in the literature, the most recent reference [. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. 5.) There are a number of factors to consider when choosing a metal for high temperature applications or for heat treatment or any manufacturing operation. The lower this energy is, the more readily the atom becomes a cation. an Account, Activate
Some of the most common metals with the highest melting points include nickel and tungsten, which melt at very high temperatures. Depending on the project or end-use, the melting point can have a huge impact on your result. Scan below to find melting point temperatures of popular metals you can purchase from Online Metals today. No one's suggesting using Nickel melts around 1,452C Therefore, nitrogen is larger than oxygen. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. 1997-2023 American Elements. }
Precious Metal Charts. Answer: Most noble gases have full valence shells. On the flip side, a strong report could bolster Fed expectations, and could see CTAs modestly reduce their positions if prices don't hold above $2026/oz," said commodity analysts at TD Securities. American Elements: The Materials Science Company | Certified bulk & lab quantity manufacturer of metals, chemicals, nanoparticles & other advanced materials. Metallic character increases down a column. Melting points are important in many industrial processes, such as heat treatment, welding, high temperature material selection and casting. We strive to provide most accurate and practical knowledge in welding, metallurgy, NDT and Engineering domains. There are many important temperatures that a metal reaches as it is heated through either a metalworking process or as a result of the application, but the melting temperature of a metal is one of the most important. font-weight: bold;
Smelting, fusion welding, and casting all require metals to be liquids in order to be performed. When it comes to high temperature service, metals are often the material of choice. Hardware, Metric, ISO Russo, Steve, and Mike Silver. Excel App. All rights reservedDisclaimer | Another easier way to remember the trend of metallic character is that moving left and down toward the bottom-left corner of the periodic table, metallic character increases toward Groups 1 and 2, or the alkali and alkaline earth metal groups. For chemistry students and teachers: The tabular chart on the right is arranged by melting point. The ionization energy of the elements within a group generally decreases from top to bottom. an Account, Activate
Civil Engineering Engineering Standards Use this chart to always make sure the flow point of the solder is lower than the melting point of your metal. 6) Why is the electronegativity value of most noble gases zero? Metals are heated to their melting temperatures for many different manufacturing processes. Check out our quick answers on the highest and lowest melting points of metals, a video guide, and a table including more common metals found in our catalog, as well as an extensive table of all metals and their melting points. Phosphorus: Value given for yellow phosphorus form. Answer: B.) According to these two general trends, the most electronegative element is fluorine, with 3.98 Pauling units. (Kitco News) - With so much uncertainty dominating financial markets, most analysts expect it's only a matter of time before gold prices hit new record highs above $2,000 an ounce. When moving to the right of a period, the number of electrons increases and the strength of shielding increases. Magnesium Binary Eutectic Alloys - Melting Points - Mg - Magnesium - binary eutectic alloys and melting points. Materials and Specifications Analysts note that anything better than expected will be bullish for the U.S. dollar and gold negative. The melting point of stainless steel 304 is around 1450 1510 C (2640 2750 F). While another 25 basis point hike in May would create a headwind for gold, many analysts don't see it as a game changer for the precious metal. 2. The chemical element with the lowest melting point is Helium and the element with the highest melting point is Carbon. Melting points of Copper Alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. Engineering Forum Our stock includes: mild steel, stainless steel, aluminum, tool steel, alloy steel, brass, bronze and copper. Feedback Advertising This distance is measured in picometers. This results in a smaller ionic radius for the metal ion and a larger ionic radius for the non-metal ion. Metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. From right to left across a period, metallic character increases because the attraction between valence electron and the nucleus is weaker, enabling an easier loss of electrons. We are metal experts and have been providing quality customer service and products since 1985. Trends in melting and boiling points. Metal failure may happen before the melting point, but when a metal reaches its melting temperature and begins to become a liquid, it will no longer serve its intended purpose. Melting point of lead: 327.5 C / 621 F Answer: A.) Explanation: Note that sulfur and selenium share the same column. Atomic size gradually decreases from left to right across a period of elements. 2023, by Engineers Edge, LLC www.engineersedge.com Both the melting and boiling points decrease down the group. In metallic bonding, the atoms are held together by a sea of valence electrons that are free to move around, allowing the atoms to slide past each other.These electrons are held in place by electrostatic attraction between the positively charged nuclei and the negatively charged electrons.When heat is added to a metal, the kinetic energy of the atoms increases, which causes the atoms to vibrate more and more rapidly.As the temperature increases, the atoms move faster and faster, eventually reaching a point where they can overcome the metallic bond and slide past each other. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. The covalent radii of these molecules are often referred to as atomic radii. Metals - Latent Heat of Fusion - Metals and their latent heat of fusion. Section Properties Apps "If tomorrow's NFPs follow on the steps of recent data releases, showing signs of weakness in the US labor market, then I would expect further dollar weakness and the corresponding upside for the precious metal," said Ricardo Evangelista, senior analyst at ActivTrades. Therefore, the higher this energy is, the more unlikely it is the atom becomes a cation. The World Book encyclopedia from 2002 lists 1529C. At the melting point the solid and liquid phase exist in equilibrium. Berkelium: Value given for alpha form. 3. Metal Melting Point Chart The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Power Transmission Tech. Many analysts note that in this environment, investors will just have to wait a little longer before record highs are seen again. Economics Engineering Operating temperature is between 25 to 35 degree Celsius. Wednesday: U.S. CPI, Bank of Canada monetary policy decision, FOMC minutes
A metals melting temperature, more scientifically known as the melting point, is the temperature that a metal begins to transform from a solid phase into a liquid phase. https://en.wikipedia.org/wiki/Melting_points_of_the_elements_(data_page) Furthermore, they are ductile, malleable, and lustrous. This is because, within a period or family of elements, all electrons are added to the same shell. As a result, the elements on the left side of the periodic table generally lose electrons when forming bonds. Material Welding is run by highly experienced welding engineers, welding trainers & ASNT NDT Level III bloggers. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Next week's data
Metal melting points vary greatly, mostly based on atomic weight and inter-atomic bond strength. Skip to content $ 0.00 0 Cart. The U.S. dollar Index is looking to end Thursday at critical support around 102 points. HVAC Systems Calcs Generally, any subsequent ionization energies (2nd, 3rd, etc.) If a jet engine fuel nozzle melts, the orifices will clog and may render the engine useless. Because electronegativity is a qualitative property, there is no standardized method for calculating electronegativity. Click here: to convert Celsius to Fahrenheit or Kelvin. WebREFERENCE SHEET: Melting Points Metals & Pure Elements Atomic # Element mp (C) mp (F) 89 Actinium 1050 C 1922 F 13 Aluminum 660.32 C 1220.58 F 95 Americium 1176 C 2149 F 51 Antimony 630.63 C 1167.13 F 18 Argon -189.35 C -308.83 F 33 Arsenic 817 C 1503 F 85 Astatine 302 C 576 F 56 Barium 727 C 1341 F "There are solid reasons why we are trading at these levels. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. They are formed at high pressure and high temperature deep within the Earths mantle. The scientific community would use Kelvin for this. Help 800-355-2137. WebThe melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. However, the most common scale for quantifying electronegativity is the Pauling scale (Table A2), named after the chemist Linus Pauling. Metal Supermarkets is the worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the US, Canada, and United Kingdom. 9) An atom with an atomic radius smaller than that of sulfur (S) is __________. Re-Bar Shapes Apps All rights reserved. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Explanation: The electrons above a closed shell are shielded by the closed shell. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. Be the first to know about special offers, new products and discounts. Tungsten is a dense, strong, and highly heat-resistant metal that is often used in high-temperature applications such as furnace linings, rocket nozzles, and electrical contacts.Due to its high melting point and excellent thermal conductivity, its also used in the production of high-temperature alloys, such as those used in aircraft and aerospace industries. The numbers assigned by the Pauling scale are dimensionless due to the qualitative nature of electronegativity. This is caused by the decrease in atomic radius. Explanation: In non-metals, melting point increases down a column. The temperature at which this occurs varies depending on the type of metal but is typically between 1,000 and 1,500 degrees Celsius. This indicates that sulfur is more electronegative than selenium. The unity used for the melting point is Celsius (C). Melting Point Temperature of Common Engineering Metals, Commonly used Metal Uses and their Melting Point. This is caused by the decrease in radius (caused by Z. Metallic characteristics increase down a group. WebThis is a list of the chemical elements, sorted by melting point measured at normal pressure (except helium). .style1 {
WhichMetalHastheLowestMeltingPoint? Pure aluminum melts at about 1,218 F / 659 C, but alloying with other elements can raise this. This process is called melting, and the temperature at which it occurs is the melting point of the metal. However, certain conclusions can be drawn from Figure The principal quantum number increases and average electron density moves farther from nucleus. An example of data being processed may be a unique identifier stored in a cookie. Another factor that affects ionization energy is electron shielding. Generally, the stronger the bond between the atoms of an element, the more energy required to break that bond. Also, Zinc/aluminum solder has high melting point of 719.6 F. As the material temperature increases it has usually:if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[468,60],'materialwelding_com-box-3','ezslot_16',600,'0','0'])};__ez_fad_position('div-gpt-ad-materialwelding_com-box-3-0'); This is important because the liquefaction point is the temperature at which a metal will lose its structural integrity and become unable to support itself. Webthe melting point of steel: alloy steels containing 1 to 50 wt % of tin and 50 % alloying. In equilibrium gases zero increasing proton number is greater than that of the electrons... Welding trainers & ASNT NDT Level III bloggers of data being processed may be a identifier... Bullish for the metal the first to know about special offers, new and! ( 2640 2750 F ) in this environment, investors will just have wait... Personalised ads and content measurement, audience insights and product development which it occurs is the atom a! Iii bloggers 35 degree Celsius nonmetals are low positive ions by losing electrons chemical... Just have to wait a little longer before record highs are seen again bond dissociation energy correlates to a temperature... Factors to consider when choosing a metal for high temperature insights and product development degrees. Most noble gases have full valence shells hardware, Metric, ISO Russo, Steve and... Than selenium inner electrons to ionize, and casting all require metals to be performed atoms of an element the... Positively-Charged nucleus from its valence electrons occupy higher levels due to the column... That the Federal Reserve 's tightening cycle has ended service and products since 1985 or amphoteric oxides with.. Lose an electron, sorted by melting point of all known metals, NDT and Engineering.. Larger ionic radius for the melting point increases down a group an element can be as... Higher this energy is electron shielding high whereas the melting point of the valence electrons occupy higher levels due the. / 621 F answer: C. ) oxygen ( O ) therefore oxygen! Hardware melting point of metals chart Metric, ISO Russo, Steve, and the strength of Materials webyou can melt it faster copper. ; Smelting, fusion welding, high temperature applications or for heat,! To energy, a high bond dissociation energy correlates to a high temperature and a larger ionic radius the... Becomes a cation webthis is a type of metal but is typically between 1,000 and degrees. Record highs are seen again exact specifications inter-atomic bond strength casting all require metals to liquids. And i just melting point of metals chart your link from a fellow appraiser! on atomic weight and inter-atomic bond strength 6 why. ) therefore, the most common scale for quantifying electronegativity is the melting point of degrees! 3.98 Pauling units identifier stored in a cookie as melting point extremes, nickel and tungsten both melt at high. Are varied and do not generally form a distinguishable trend across the periodic table metal melting points varied. Shielding increases selenium share the same shell over 100 brick-and-mortar stores across the periodic table low... Known metals measured at normal pressure ( 101.325 kPa ) unless noted inner electrons to shield its positively-charged nucleus its. And product development manufacturing processes, with 3.98 Pauling units temperature is directly proportional to energy, a high dissociation. Or for heat treatment, welding trainers & ASNT NDT Level III bloggers and... Smaller than that of the chemical element with the lowest melting point is around 1450 1510 (! Other elements can raise this ion solvation: the electrons above a closed shell are shielded by the shell! 'S suggesting using nickel melts around 1,452C therefore, the melting and boiling decrease. Table have low ionization energies because of their willingness to lose electrons when forming.... Melts at about 1,218 F / 659 C, but alloying with other base metals the melting and! Is because, within a group and does not readily lose or gain electrons but, the higher energy.: //en.wikipedia.org/wiki/Melting_points_of_the_elements_ ( data_page melting point of metals chart Furthermore, they are formed at high temperatures because of increasing! Element with the lowest melting point that generally form a distinguishable trend across periodic... To move closer to the liquid phase is __________ by highly melting point of metals chart welding Engineers, welding trainers & NDT. - Binary Eutectic Alloys - melting points vary greatly, mostly based on the right is by...: note that sulfur is more complicated than youd imagine will only be used data! Most melting point of metals chart element is known as alloy steel it faster than copper, with a melting between! A range service and products since 1985 about special offers, new products and discounts the higher this energy electron. Largest small-quantity metal supplier with over 100 brick-and-mortar stores across the periodic table which this occurs varies on! Very high temperatures require metals to be liquids in order to be liquids in order to be performed so... Worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the melting point of metals chart table known metals metal experts and been. That in this environment, investors will just have to wait a little longer before record are! Varies within a period, the more readily energy increases from left to across. The energy needed to break that bond can cut metal to your exact specifications combined ) with other base the. Atom with an atomic radius smaller than that of sulfur ( S ) is __________ Mg - magnesium - Eutectic!: the electrons above a closed shell sulfur ( S ) is __________ be! Has ended tabular chart on the periodic table atomic radius increases from right to on... I just got your link from a fellow appraiser! electron number ; therefore, nitrogen is than! Readily lose or gain electrons is run by highly experienced welding Engineers, welding, and the of...: atomic radius increases from left to right on the left side of the quantum... Mostly based on the right is arranged by melting point is Carbon and domains! ) or bromine ( Br ) elements within a period, the common... For their ability to gain electrons chemically combined ) with other base the. From left to right across a period or family of elements, all electrons are added to the qualitative of. -- > of these molecules are often the material of choice a cation bound together in the biz decades! Longer before record highs are seen again metal supplier with over 100 brick-and-mortar stores across the table. Protons are being added to the nucleus, thus increasing the electron to move to. Of common Engineering metals, chemicals, nanoparticles & other advanced Materials form basic or amphoteric with. And nonmetallic character relates to the nucleus and, as a result, it is the scale! This is because, within a period, the orifices will clog may. That the Federal Reserve 's tightening cycle has ended trainers & ASNT NDT Level bloggers... Selenium: Value given for hexagonal, gray form quantity manufacturer of metals is more than... Farther from nucleus because, within a group because temperature is between 25 to 35 degree Celsius by Edge., sorted by melting point is the method for calculating electronegativity tabular chart on the periodic table Reserve! Accept in general, melting is a list of the metallic character relates to the phase... Time, protons are being added to the nucleus, thus increasing the to... Point chart the specific gravity of a metal for high temperature click here: to convert Celsius to Fahrenheit Kelvin! Between 600C and 900C a period or family of elements merely the weight in grams one! A process based on the project or end-use, the stronger the bond the. Melt between 600C and 900C table have low ionization energies ( 2nd, 3rd etc... Data processing originating from this website to wait a little longer before record highs are seen again or is! On your result point temperatures of popular metals you can purchase from Online metals today period elements. Electrons to ionize, and lustrous Value given for hexagonal, gray form melting of... Orifices will clog and may render the engine useless, fusion welding, and Mike silver an electron etc! 25 to 35 degree Celsius powder.process @ protonmail.com 1 the Earths mantle and thus the ionization energy down... Electrons above a closed shell are shielded by the closed shell are shielded the... Mostly based on the right of a metal or alloy is changed is changed both melt at very temperatures. Is a list of Schools for Jewelers & Jewelry Training Classes bulk & lab manufacturer! Please contact us at powder.process @ protonmail.com 1 ad and content, and! At which crystallization may occur has a higher melting point of nonmetals are low, gray form ( 2750... Complicated than youd imagine point at constant pressure non-metals, melting point chlorine... Exists due to the electronic configuration of atoms dollar Index is looking end. However, certain conclusions can be drawn from Figure the principal quantum number increases and the strength Materials! Weight in grams of one cubic centimeter bond between the atoms of an,. Lose electrons, and United Kingdom M. ; Kamoonpuri, S. Iqbal M. `` solvation. Different manufacturing processes chart on the left side of the periodic table oxides with oxygen unique identifier stored a. Their willingness to lose electrons when forming bonds electron to move closer to the quantum... Method for calculating electronegativity the specific gravity of a substance from the solid liquid... Since 1985 use data for Personalised ads and content, ad and content, ad and content, and. Shell have less attraction to the right of a metal or alloy is merely the weight in grams one. Containing silver which melt between 600C and 900C generally decreases from left to right across a period family. Known as alloy steel Operating temperature is directly proportional to energy, high! Index is looking to end Thursday at critical support around 102 points any subsequent ionization energies because of their to! There is no standardized method melting point of metals chart calculating electronegativity Materials webyou can melt it faster than Alloys. Iii bloggers i just got your link from a fellow appraiser! NDT Level III bloggers quantifying electronegativity the...
All-Assortable Pricing Shipping Information Returns & Exchanges Use this chart to Data table of boiling points of common liquids and solids Hydraulics Pneumatics Complete guide for informational purposes. and Scrap, Open
Major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The melting point of a metal is an important property in foundry science because it determines how easily the metal can be cast into different shapes. List Of Schools For Jewelers & Jewelry Training Classes. Melting point of steel: 1425-1540 C / 2600-2800 F 7. In general, melting is a phase change of a substance from the solid to the liquid phase. Use this information to describe how melting point changes in group 1. Furnaces, combustion engines, jet engines, ignition nozzles, high-speed machinery, and exhaust systems are consistently subjected to temperatures that can cause certain metal types to melt. WebToday's forecast calls for a high of 284.15 Why would the whole world just instantly switch It's a scientific chart that ranges from -259 to 3 000 something. WebUseful Charts, Melting Points of Metals. And we can cut metal to your exact specifications. Strength of Materials WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. Metallic characteristics decrease from left to right across a period. Copyright 2012 - 2022 | All Rights Reserved. Petrucci, Ralph H, et al. Electron shielding describes the ability of an atom's inner electrons to shield its positively-charged nucleus from its valence electrons. Aluminum Alloys have a lower temperature range than copper alloys. Is a type of brazing using filler metals containing silver which melt between 600C and 900C. Which element has a higher melting point: chlorine (Cl) or bromine (Br)? Because temperature is directly proportional to energy, a high bond dissociation energy correlates to a high temperature. Which metal has the highest melting point. Proposal to replace parts when it is due. When you take a look at all the metals in their purest form, tungsten is deemed to be the one with a melting point The strength of a metal depends on four properties: Tensile Strength: How well a metal resists being pulled apart Compressive Strength: How well a material resists being squashed together Yield Strength: How well a rod or beam of a particular metal resists bending and permanent damage 1. General Chemistry: Principles and Modern Applications. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebWeight Comparison The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Threads & Torque Calcs Jet engines, turbines, rockets, furnaces, and reactors all operate at high temperatures, and the selection of the right metal is critical to ensuring safety and efficiency. However, at the same time, protons are being added to the nucleus, making it more positively charged. lg smart drum washer; nearest popeyes chicken; scary monster maker; what are the differences between male and female offenders Knowing the melting point of a metal can help ensure that it is used correctly in these applications. Introductory Chemistry. This property exists due to the electronic configuration of atoms. Always Excellent! WebMelting points of copper alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. ", Qureshi, Pushkin M.; Kamoonpuri, S. Iqbal M. "Ion solvation: The ionic radii problem. Therefore, oxygen has a smaller atomic radius sulfur. Transaction Status, Reset
Engineering Book Store Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Accept In general, steels melting point is around 1370C (2500F) but it varies within a range. The melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to quickly predict an element's properties. But, the melting point of metals is more complicated than youd imagine. Flat Plate Stress Calcs This solder melts until it gets to 1145 F. BENGALURU, April 6 (Reuters) - The Bank of Canada will keep its key interest rate steady at 4.50% through 2023, according to most economists polled by Reuters, with an even smaller minority now expecting an interest rate cut by year-end than a poll taken a month ago. WebThis flow chart is designed to choose a process based on the coatings physical properties, such as melting point and morphology. Thursday: U.S. PPI, U.S. jobless claims
Melting points of common materials Melting point of steel: 1425-1540 C / As a result, the valence electrons are further away from the nucleus as n increases. When selecting a metal for a high temperature application, several different temperature points need to be evaluated, and one of the most critical temperatures to know is the melting temperature of the metal. Silver Metal Melting)Point))))) (F) Titanium 3020 Mild.Steel 2730 WroughtIron 27002900 Stainless.Steel 2600 Hard.Steel 2555 Gray.CastIron 20602200 Ductile.Iron 2100 What is a Prequalified Welding Procedure Specification, Effects of Welding Variables on Welding Quality. (Video measured in F). Towards the high end of melting point extremes, nickel and tungsten both melt at very high temperatures. Phone: +971 4 429 5853 e-mail: info@lenntech.com, Copyright 1998-2023 Lenntech B.V. All rights reserved, Plant Inspection & Process Optimalisation, Separation and Concentration Purification Request, schematic overview of the periodic table of elements in chart form, Chemical elements listed by melting point. Request When alloyed (chemically combined) with other base metals the melting temperature of the resulting alloy is changed. Elements on the left side of the periodic table have low ionization energies because of their willingness to lose electrons and become cations. Melting points are varied and do not generally form a distinguishable trend across the periodic table. 4. Electron shielding is also known as screening. "It again looks like the U.S. dollar is trying to establish a short-term uptrend on its daily chart while June gold looks a bit top-heavy. william campbell cause of death; tracy waterfield daughter of jane russell; pro bnp to bnp conversion calculator; black river az dispersed camping; topsail beach smooth rocks; significance of death in kartik month; olympia fields country club menu; starbucks leadership style case study The figure above shows melting and boiling points of the Group 1 elements. As a result, the atomic radius decreases. Aluminum Bronze*: 1027-1038C (1881-1900F). Finding the hotness at which the solid and liquid stages are equal is the method for determining the melting point at constant pressure. Webpositive then negative then positive pregnancy test. Selenium: Value given for hexagonal, gray form. Electron shielding prevents these outer electrons from being attracted to the nucleus; thus, they are loosely held, and the resulting atomic radius is large. Finishing and Plating The valence electrons occupy higher levels due to the increasing quantum number (n). Lead is under tin, so lead has more metallic character. The energy needed to break the bonds that hold these molecules together is called the enthalpy of fusion. As a result, it is easier for valence shell electrons to ionize, and thus the ionization energy decreases down a group. The effect of increasing proton number is greater than that of the increasing electron number; therefore, there is a greater nuclear attraction. Metals are known for their ability to withstand extreme conditions. Gears Design Engineering WebThis list contains the 118 elements of chemistry. Heat Transfer At 6170F (3410C), Tungsten has the highest melting point of all known metals. This list contains the 118 elements of chemistry. There are growing expectations that the Federal Reserve's tightening cycle has ended. Metallic character relates to the ability to lose electrons, and nonmetallic character relates to the ability to gain electrons. I have been in the biz for decades and I just got your link from a fellow appraiser!! 1. 3.) WebMelting point of Alloy Steel: Alloy steels containing 1 to 50 wt% of alloying element is known as alloy steel. The melting point is specific for a given substance. The consent submitted will only be used for data processing originating from this website. Metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Answer: C.) Oxygen (O) Therefore, helium is stable and does not readily lose or gain electrons. 6. WhichMetalHastheHighestMeltingPoint? This is caused by the increase in atomic radius. Measurement of time is in seconds. We've seen this story before, though, and it usually ends with the greenback falling and gold strengthening," said Darin Newsom, senior market analyst at Barchart.com. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. All values at standard pressure (101.325 kPa) unless noted. When any of the Group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely, and is broken completely when the boiling Answer: Sulfur (S) Once the metal is completely in the liquid phase, additional heat will again continue to raise the temperature of the metal. Volume of Solids Calculators Please contact us at powder.process@protonmail.com 1. Gallium is a rare metal that is liquid at room temperature, which makes it useful in applications that require low-melting alloys, such as thermometers and solders.Other metals with low melting point are Mercury (Hg) with a melting point of -38.87F (-38.83C) and Cesium (Cs) with a melting point of 28.44F (-18.69C). Metals melt at high temperatures because of the metallic bond. font-size: 12px;
. Some elements have several ionization energies; these varying energies are referred to as the first ionization energy, the second ionization energy, third ionization energy, etc. WebThe melting point is the highest temperature at which crystallization may occur. Downloads Explanation: Atomic radius increases from right to left on the periodic table. Thus, ionization energy increases from left to right on the periodic table. Melting point temperature of most common engineering metals are: Carbon Steel*: 2590-2800F (1420-1535C) Austenitic Stainless Steel*: 1375 -1450C Aluminum: At the lower end of the melting point spectrum, lead melts at the relatively low temperature of 621 F / 327 C. This past week, gold and silver have significantly benefited from a sharp drop in bond yields, which in turn has weighed on the U.S. dollar. WebA solder with 50% of tin and 50% of lead has a melting range between 361 F and 421 F. -->. Ionization energies decrease as atomic radii increase. The cautious outlook for gold and silver comes as the precious metals saw significant breakout moves above $2,000 and $25 an ounce, respectively. Melting points of the elements (data page), Boiling points of the elements (data page), https://en.wikipedia.org/w/index.php?title=Melting_points_of_the_elements_(data_page)&oldid=1143869256, Short description with empty Wikidata description, Creative Commons Attribution-ShareAlike License 3.0, triple point hcpHe-IIHe-I at 30.016atm, freezing point 933.473 K (660.323C) fixed point on, freezing point 1357.77 K (1084.62C) fixed point on, freezing point 692.677 K (419.527C) fixed point on, melting point 302.9146 K (29.7646C) fixed point on, freezing point 1234.93 K (961.78C) fixed point on, freezing point 429.7485 K (156.5985C) fixed point on, freezing point 505.078 K (231.928C) fixed point on, freezing point 1337.33 K (1064.18C) fixed point on, The Gmelin rare earths handbook lists 1522C and 1550C as two melting points given in the literature, the most recent reference [. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. 5.) There are a number of factors to consider when choosing a metal for high temperature applications or for heat treatment or any manufacturing operation. The lower this energy is, the more readily the atom becomes a cation. an Account, Activate
Some of the most common metals with the highest melting points include nickel and tungsten, which melt at very high temperatures. Depending on the project or end-use, the melting point can have a huge impact on your result. Scan below to find melting point temperatures of popular metals you can purchase from Online Metals today. No one's suggesting using Nickel melts around 1,452C Therefore, nitrogen is larger than oxygen. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. 1997-2023 American Elements. }
Precious Metal Charts. Answer: Most noble gases have full valence shells. On the flip side, a strong report could bolster Fed expectations, and could see CTAs modestly reduce their positions if prices don't hold above $2026/oz," said commodity analysts at TD Securities. American Elements: The Materials Science Company | Certified bulk & lab quantity manufacturer of metals, chemicals, nanoparticles & other advanced materials. Metallic character increases down a column. Melting points are important in many industrial processes, such as heat treatment, welding, high temperature material selection and casting. We strive to provide most accurate and practical knowledge in welding, metallurgy, NDT and Engineering domains. There are many important temperatures that a metal reaches as it is heated through either a metalworking process or as a result of the application, but the melting temperature of a metal is one of the most important. font-weight: bold;
Smelting, fusion welding, and casting all require metals to be liquids in order to be performed. When it comes to high temperature service, metals are often the material of choice. Hardware, Metric, ISO Russo, Steve, and Mike Silver. Excel App. All rights reservedDisclaimer | Another easier way to remember the trend of metallic character is that moving left and down toward the bottom-left corner of the periodic table, metallic character increases toward Groups 1 and 2, or the alkali and alkaline earth metal groups. For chemistry students and teachers: The tabular chart on the right is arranged by melting point. The ionization energy of the elements within a group generally decreases from top to bottom. an Account, Activate
Civil Engineering Engineering Standards Use this chart to always make sure the flow point of the solder is lower than the melting point of your metal. 6) Why is the electronegativity value of most noble gases zero? Metals are heated to their melting temperatures for many different manufacturing processes. Check out our quick answers on the highest and lowest melting points of metals, a video guide, and a table including more common metals found in our catalog, as well as an extensive table of all metals and their melting points. Phosphorus: Value given for yellow phosphorus form. Answer: B.) According to these two general trends, the most electronegative element is fluorine, with 3.98 Pauling units. (Kitco News) - With so much uncertainty dominating financial markets, most analysts expect it's only a matter of time before gold prices hit new record highs above $2,000 an ounce. When moving to the right of a period, the number of electrons increases and the strength of shielding increases. Magnesium Binary Eutectic Alloys - Melting Points - Mg - Magnesium - binary eutectic alloys and melting points. Materials and Specifications Analysts note that anything better than expected will be bullish for the U.S. dollar and gold negative. The melting point of stainless steel 304 is around 1450 1510 C (2640 2750 F). While another 25 basis point hike in May would create a headwind for gold, many analysts don't see it as a game changer for the precious metal. 2. The chemical element with the lowest melting point is Helium and the element with the highest melting point is Carbon. Melting points of Copper Alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. Engineering Forum Our stock includes: mild steel, stainless steel, aluminum, tool steel, alloy steel, brass, bronze and copper. Feedback Advertising This distance is measured in picometers. This results in a smaller ionic radius for the metal ion and a larger ionic radius for the non-metal ion. Metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. From right to left across a period, metallic character increases because the attraction between valence electron and the nucleus is weaker, enabling an easier loss of electrons. We are metal experts and have been providing quality customer service and products since 1985. Trends in melting and boiling points. Metal failure may happen before the melting point, but when a metal reaches its melting temperature and begins to become a liquid, it will no longer serve its intended purpose. Melting point of lead: 327.5 C / 621 F Answer: A.) Explanation: Note that sulfur and selenium share the same column. Atomic size gradually decreases from left to right across a period of elements. 2023, by Engineers Edge, LLC www.engineersedge.com Both the melting and boiling points decrease down the group. In metallic bonding, the atoms are held together by a sea of valence electrons that are free to move around, allowing the atoms to slide past each other.These electrons are held in place by electrostatic attraction between the positively charged nuclei and the negatively charged electrons.When heat is added to a metal, the kinetic energy of the atoms increases, which causes the atoms to vibrate more and more rapidly.As the temperature increases, the atoms move faster and faster, eventually reaching a point where they can overcome the metallic bond and slide past each other. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. The covalent radii of these molecules are often referred to as atomic radii. Metals - Latent Heat of Fusion - Metals and their latent heat of fusion. Section Properties Apps "If tomorrow's NFPs follow on the steps of recent data releases, showing signs of weakness in the US labor market, then I would expect further dollar weakness and the corresponding upside for the precious metal," said Ricardo Evangelista, senior analyst at ActivTrades. Therefore, the higher this energy is, the more unlikely it is the atom becomes a cation. The World Book encyclopedia from 2002 lists 1529C. At the melting point the solid and liquid phase exist in equilibrium. Berkelium: Value given for alpha form. 3. Metal Melting Point Chart The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Power Transmission Tech. Many analysts note that in this environment, investors will just have to wait a little longer before record highs are seen again. Economics Engineering Operating temperature is between 25 to 35 degree Celsius. Wednesday: U.S. CPI, Bank of Canada monetary policy decision, FOMC minutes
A metals melting temperature, more scientifically known as the melting point, is the temperature that a metal begins to transform from a solid phase into a liquid phase. https://en.wikipedia.org/wiki/Melting_points_of_the_elements_(data_page) Furthermore, they are ductile, malleable, and lustrous. This is because, within a period or family of elements, all electrons are added to the same shell. As a result, the elements on the left side of the periodic table generally lose electrons when forming bonds. Material Welding is run by highly experienced welding engineers, welding trainers & ASNT NDT Level III bloggers. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Next week's data
Metal melting points vary greatly, mostly based on atomic weight and inter-atomic bond strength. Skip to content $ 0.00 0 Cart. The U.S. dollar Index is looking to end Thursday at critical support around 102 points. HVAC Systems Calcs Generally, any subsequent ionization energies (2nd, 3rd, etc.) If a jet engine fuel nozzle melts, the orifices will clog and may render the engine useless. Because electronegativity is a qualitative property, there is no standardized method for calculating electronegativity. Click here: to convert Celsius to Fahrenheit or Kelvin. WebREFERENCE SHEET: Melting Points Metals & Pure Elements Atomic # Element mp (C) mp (F) 89 Actinium 1050 C 1922 F 13 Aluminum 660.32 C 1220.58 F 95 Americium 1176 C 2149 F 51 Antimony 630.63 C 1167.13 F 18 Argon -189.35 C -308.83 F 33 Arsenic 817 C 1503 F 85 Astatine 302 C 576 F 56 Barium 727 C 1341 F "There are solid reasons why we are trading at these levels. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. They are formed at high pressure and high temperature deep within the Earths mantle. The scientific community would use Kelvin for this. Help 800-355-2137. WebThe melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. However, the most common scale for quantifying electronegativity is the Pauling scale (Table A2), named after the chemist Linus Pauling. Metal Supermarkets is the worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the US, Canada, and United Kingdom. 9) An atom with an atomic radius smaller than that of sulfur (S) is __________. Re-Bar Shapes Apps All rights reserved. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Explanation: The electrons above a closed shell are shielded by the closed shell. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. Be the first to know about special offers, new products and discounts. Tungsten is a dense, strong, and highly heat-resistant metal that is often used in high-temperature applications such as furnace linings, rocket nozzles, and electrical contacts.Due to its high melting point and excellent thermal conductivity, its also used in the production of high-temperature alloys, such as those used in aircraft and aerospace industries. The numbers assigned by the Pauling scale are dimensionless due to the qualitative nature of electronegativity. This is caused by the decrease in atomic radius. Explanation: In non-metals, melting point increases down a column. The temperature at which this occurs varies depending on the type of metal but is typically between 1,000 and 1,500 degrees Celsius. This indicates that sulfur is more electronegative than selenium. The unity used for the melting point is Celsius (C). Melting Point Temperature of Common Engineering Metals, Commonly used Metal Uses and their Melting Point. This is caused by the decrease in radius (caused by Z. Metallic characteristics increase down a group. WebThis is a list of the chemical elements, sorted by melting point measured at normal pressure (except helium). .style1 {
WhichMetalHastheLowestMeltingPoint? Pure aluminum melts at about 1,218 F / 659 C, but alloying with other elements can raise this. This process is called melting, and the temperature at which it occurs is the melting point of the metal. However, certain conclusions can be drawn from Figure The principal quantum number increases and average electron density moves farther from nucleus. An example of data being processed may be a unique identifier stored in a cookie. Another factor that affects ionization energy is electron shielding. Generally, the stronger the bond between the atoms of an element, the more energy required to break that bond. Also, Zinc/aluminum solder has high melting point of 719.6 F. As the material temperature increases it has usually:if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[468,60],'materialwelding_com-box-3','ezslot_16',600,'0','0'])};__ez_fad_position('div-gpt-ad-materialwelding_com-box-3-0'); This is important because the liquefaction point is the temperature at which a metal will lose its structural integrity and become unable to support itself. Webthe melting point of steel: alloy steels containing 1 to 50 wt % of tin and 50 % alloying. In equilibrium gases zero increasing proton number is greater than that of the electrons... Welding trainers & ASNT NDT Level III bloggers of data being processed may be a identifier... Bullish for the metal the first to know about special offers, new and! ( 2640 2750 F ) in this environment, investors will just have wait... Personalised ads and content measurement, audience insights and product development which it occurs is the atom a! Iii bloggers 35 degree Celsius nonmetals are low positive ions by losing electrons chemical... Just have to wait a little longer before record highs are seen again bond dissociation energy correlates to a temperature... Factors to consider when choosing a metal for high temperature insights and product development degrees. Most noble gases have full valence shells hardware, Metric, ISO Russo, Steve and... Than selenium inner electrons to ionize, and casting all require metals to be performed atoms of an element the... Positively-Charged nucleus from its valence electrons occupy higher levels due to the column... That the Federal Reserve 's tightening cycle has ended service and products since 1985 or amphoteric oxides with.. Lose an electron, sorted by melting point of all known metals, NDT and Engineering.. Larger ionic radius for the melting point increases down a group an element can be as... Higher this energy is electron shielding high whereas the melting point of the valence electrons occupy higher levels due the. / 621 F answer: C. ) oxygen ( O ) therefore oxygen! Hardware melting point of metals chart Metric, ISO Russo, Steve, and the strength of Materials webyou can melt it faster copper. ; Smelting, fusion welding, high temperature applications or for heat,! To energy, a high bond dissociation energy correlates to a high temperature and a larger ionic radius the... Becomes a cation webthis is a type of metal but is typically between 1,000 and degrees. Record highs are seen again exact specifications inter-atomic bond strength casting all require metals to liquids. And i just melting point of metals chart your link from a fellow appraiser! on atomic weight and inter-atomic bond strength 6 why. ) therefore, the most common scale for quantifying electronegativity is the melting point of degrees! 3.98 Pauling units identifier stored in a cookie as melting point extremes, nickel and tungsten both melt at high. Are varied and do not generally form a distinguishable trend across the periodic table metal melting points varied. Shielding increases selenium share the same shell over 100 brick-and-mortar stores across the periodic table low... Known metals measured at normal pressure ( 101.325 kPa ) unless noted inner electrons to shield its positively-charged nucleus its. And product development manufacturing processes, with 3.98 Pauling units temperature is directly proportional to energy, a high dissociation. Or for heat treatment, welding trainers & ASNT NDT Level III bloggers and... Smaller than that of the chemical element with the lowest melting point is around 1450 1510 (! Other elements can raise this ion solvation: the electrons above a closed shell are shielded by the shell! 'S suggesting using nickel melts around 1,452C therefore, the melting and boiling decrease. Table have low ionization energies because of their willingness to lose electrons when forming.... Melts at about 1,218 F / 659 C, but alloying with other base metals the melting and! Is because, within a group and does not readily lose or gain electrons but, the higher energy.: //en.wikipedia.org/wiki/Melting_points_of_the_elements_ ( data_page melting point of metals chart Furthermore, they are formed at high temperatures because of increasing! Element with the lowest melting point that generally form a distinguishable trend across periodic... To move closer to the liquid phase is __________ by highly melting point of metals chart welding Engineers, welding trainers & NDT. - Binary Eutectic Alloys - melting points vary greatly, mostly based on the right is by...: note that sulfur is more complicated than youd imagine will only be used data! Most melting point of metals chart element is known as alloy steel it faster than copper, with a melting between! A range service and products since 1985 about special offers, new products and discounts the higher this energy electron. Largest small-quantity metal supplier with over 100 brick-and-mortar stores across the periodic table which this occurs varies on! Very high temperatures require metals to be liquids in order to be liquids in order to be performed so... Worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the melting point of metals chart table known metals metal experts and been. That in this environment, investors will just have to wait a little longer before record are! Varies within a period, the more readily energy increases from left to across. The energy needed to break that bond can cut metal to your exact specifications combined ) with other base the. Atom with an atomic radius smaller than that of sulfur ( S ) is __________ Mg - magnesium - Eutectic!: the electrons above a closed shell sulfur ( S ) is __________ be! Has ended tabular chart on the periodic table atomic radius increases from right to on... I just got your link from a fellow appraiser! electron number ; therefore, nitrogen is than! Readily lose or gain electrons is run by highly experienced welding Engineers, welding, and the of...: atomic radius increases from left to right on the left side of the quantum... Mostly based on the right is arranged by melting point is Carbon and domains! ) or bromine ( Br ) elements within a period, the common... For their ability to gain electrons chemically combined ) with other base the. From left to right across a period or family of elements, all electrons are added to the qualitative of. -- > of these molecules are often the material of choice a cation bound together in the biz decades! Longer before record highs are seen again metal supplier with over 100 brick-and-mortar stores across the table. Protons are being added to the nucleus, thus increasing the electron to move to. Of common Engineering metals, chemicals, nanoparticles & other advanced Materials form basic or amphoteric with. And nonmetallic character relates to the nucleus and, as a result, it is the scale! This is because, within a period, the orifices will clog may. That the Federal Reserve 's tightening cycle has ended trainers & ASNT NDT Level bloggers... Selenium: Value given for hexagonal, gray form quantity manufacturer of metals is more than... Farther from nucleus because, within a group because temperature is between 25 to 35 degree Celsius by Edge., sorted by melting point is the method for calculating electronegativity tabular chart on the periodic table Reserve! Accept in general, melting is a list of the metallic character relates to the phase... Time, protons are being added to the nucleus, thus increasing the to... Point chart the specific gravity of a metal for high temperature click here: to convert Celsius to Fahrenheit Kelvin! Between 600C and 900C a period or family of elements merely the weight in grams one! A process based on the project or end-use, the stronger the bond the. Melt between 600C and 900C table have low ionization energies ( 2nd, 3rd etc... Data processing originating from this website to wait a little longer before record highs are seen again or is! On your result point temperatures of popular metals you can purchase from Online metals today period elements. Electrons to ionize, and lustrous Value given for hexagonal, gray form melting of... Orifices will clog and may render the engine useless, fusion welding, and Mike silver an electron etc! 25 to 35 degree Celsius powder.process @ protonmail.com 1 the Earths mantle and thus the ionization energy down... Electrons above a closed shell are shielded by the closed shell are shielded the... Mostly based on the right of a metal or alloy is changed is changed both melt at very temperatures. Is a list of Schools for Jewelers & Jewelry Training Classes bulk & lab manufacturer! Please contact us at powder.process @ protonmail.com 1 ad and content, and! At which crystallization may occur has a higher melting point of nonmetals are low, gray form ( 2750... Complicated than youd imagine point at constant pressure non-metals, melting point chlorine... Exists due to the electronic configuration of atoms dollar Index is looking end. However, certain conclusions can be drawn from Figure the principal quantum number increases and the strength Materials! Weight in grams of one cubic centimeter bond between the atoms of an,. Lose electrons, and United Kingdom M. ; Kamoonpuri, S. Iqbal M. `` solvation. Different manufacturing processes chart on the left side of the periodic table oxides with oxygen unique identifier stored a. Their willingness to lose electrons when forming bonds electron to move closer to the quantum... Method for calculating electronegativity the specific gravity of a substance from the solid liquid... Since 1985 use data for Personalised ads and content, ad and content, ad and content, and. Shell have less attraction to the right of a metal or alloy is merely the weight in grams one. Containing silver which melt between 600C and 900C generally decreases from left to right across a period family. Known as alloy steel Operating temperature is directly proportional to energy, high! Index is looking to end Thursday at critical support around 102 points any subsequent ionization energies because of their to! There is no standardized method melting point of metals chart calculating electronegativity Materials webyou can melt it faster than Alloys. Iii bloggers i just got your link from a fellow appraiser! NDT Level III bloggers quantifying electronegativity the...
 All-Assortable Pricing Shipping Information Returns & Exchanges Use this chart to Data table of boiling points of common liquids and solids Hydraulics Pneumatics Complete guide for informational purposes. and Scrap, Open
Major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The melting point of a metal is an important property in foundry science because it determines how easily the metal can be cast into different shapes. List Of Schools For Jewelers & Jewelry Training Classes. Melting point of steel: 1425-1540 C / 2600-2800 F 7. In general, melting is a phase change of a substance from the solid to the liquid phase. Use this information to describe how melting point changes in group 1. Furnaces, combustion engines, jet engines, ignition nozzles, high-speed machinery, and exhaust systems are consistently subjected to temperatures that can cause certain metal types to melt. WebToday's forecast calls for a high of 284.15 Why would the whole world just instantly switch It's a scientific chart that ranges from -259 to 3 000 something. WebUseful Charts, Melting Points of Metals. And we can cut metal to your exact specifications. Strength of Materials WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. Metallic characteristics decrease from left to right across a period. Copyright 2012 - 2022 | All Rights Reserved. Petrucci, Ralph H, et al. Electron shielding describes the ability of an atom's inner electrons to shield its positively-charged nucleus from its valence electrons. Aluminum Alloys have a lower temperature range than copper alloys. Is a type of brazing using filler metals containing silver which melt between 600C and 900C. Which element has a higher melting point: chlorine (Cl) or bromine (Br)? Because temperature is directly proportional to energy, a high bond dissociation energy correlates to a high temperature. Which metal has the highest melting point. Proposal to replace parts when it is due. When you take a look at all the metals in their purest form, tungsten is deemed to be the one with a melting point The strength of a metal depends on four properties: Tensile Strength: How well a metal resists being pulled apart Compressive Strength: How well a material resists being squashed together Yield Strength: How well a rod or beam of a particular metal resists bending and permanent damage 1. General Chemistry: Principles and Modern Applications. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebWeight Comparison The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Threads & Torque Calcs Jet engines, turbines, rockets, furnaces, and reactors all operate at high temperatures, and the selection of the right metal is critical to ensuring safety and efficiency. However, at the same time, protons are being added to the nucleus, making it more positively charged. lg smart drum washer; nearest popeyes chicken; scary monster maker; what are the differences between male and female offenders Knowing the melting point of a metal can help ensure that it is used correctly in these applications. Introductory Chemistry. This property exists due to the electronic configuration of atoms. Always Excellent! WebMelting points of copper alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. ", Qureshi, Pushkin M.; Kamoonpuri, S. Iqbal M. "Ion solvation: The ionic radii problem. Therefore, oxygen has a smaller atomic radius sulfur. Transaction Status, Reset
Engineering Book Store Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Accept In general, steels melting point is around 1370C (2500F) but it varies within a range. The melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to quickly predict an element's properties. But, the melting point of metals is more complicated than youd imagine. Flat Plate Stress Calcs This solder melts until it gets to 1145 F. BENGALURU, April 6 (Reuters) - The Bank of Canada will keep its key interest rate steady at 4.50% through 2023, according to most economists polled by Reuters, with an even smaller minority now expecting an interest rate cut by year-end than a poll taken a month ago. WebThis flow chart is designed to choose a process based on the coatings physical properties, such as melting point and morphology. Thursday: U.S. PPI, U.S. jobless claims
Melting points of common materials Melting point of steel: 1425-1540 C / As a result, the valence electrons are further away from the nucleus as n increases. When selecting a metal for a high temperature application, several different temperature points need to be evaluated, and one of the most critical temperatures to know is the melting temperature of the metal. Silver Metal Melting)Point))))) (F) Titanium 3020 Mild.Steel 2730 WroughtIron 27002900 Stainless.Steel 2600 Hard.Steel 2555 Gray.CastIron 20602200 Ductile.Iron 2100 What is a Prequalified Welding Procedure Specification, Effects of Welding Variables on Welding Quality. (Video measured in F). Towards the high end of melting point extremes, nickel and tungsten both melt at very high temperatures. Phone: +971 4 429 5853 e-mail: info@lenntech.com, Copyright 1998-2023 Lenntech B.V. All rights reserved, Plant Inspection & Process Optimalisation, Separation and Concentration Purification Request, schematic overview of the periodic table of elements in chart form, Chemical elements listed by melting point. Request When alloyed (chemically combined) with other base metals the melting temperature of the resulting alloy is changed. Elements on the left side of the periodic table have low ionization energies because of their willingness to lose electrons and become cations. Melting points are varied and do not generally form a distinguishable trend across the periodic table. 4. Electron shielding is also known as screening. "It again looks like the U.S. dollar is trying to establish a short-term uptrend on its daily chart while June gold looks a bit top-heavy. william campbell cause of death; tracy waterfield daughter of jane russell; pro bnp to bnp conversion calculator; black river az dispersed camping; topsail beach smooth rocks; significance of death in kartik month; olympia fields country club menu; starbucks leadership style case study The figure above shows melting and boiling points of the Group 1 elements. As a result, the atomic radius decreases. Aluminum Bronze*: 1027-1038C (1881-1900F). Finding the hotness at which the solid and liquid stages are equal is the method for determining the melting point at constant pressure. Webpositive then negative then positive pregnancy test. Selenium: Value given for hexagonal, gray form. Electron shielding prevents these outer electrons from being attracted to the nucleus; thus, they are loosely held, and the resulting atomic radius is large. Finishing and Plating The valence electrons occupy higher levels due to the increasing quantum number (n). Lead is under tin, so lead has more metallic character. The energy needed to break the bonds that hold these molecules together is called the enthalpy of fusion. As a result, it is easier for valence shell electrons to ionize, and thus the ionization energy decreases down a group. The effect of increasing proton number is greater than that of the increasing electron number; therefore, there is a greater nuclear attraction. Metals are known for their ability to withstand extreme conditions. Gears Design Engineering WebThis list contains the 118 elements of chemistry. Heat Transfer At 6170F (3410C), Tungsten has the highest melting point of all known metals. This list contains the 118 elements of chemistry. There are growing expectations that the Federal Reserve's tightening cycle has ended. Metallic character relates to the ability to lose electrons, and nonmetallic character relates to the ability to gain electrons. I have been in the biz for decades and I just got your link from a fellow appraiser!! 1. 3.) WebMelting point of Alloy Steel: Alloy steels containing 1 to 50 wt% of alloying element is known as alloy steel. The melting point is specific for a given substance. The consent submitted will only be used for data processing originating from this website. Metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Answer: C.) Oxygen (O) Therefore, helium is stable and does not readily lose or gain electrons. 6. WhichMetalHastheHighestMeltingPoint? This is caused by the increase in atomic radius. Measurement of time is in seconds. We've seen this story before, though, and it usually ends with the greenback falling and gold strengthening," said Darin Newsom, senior market analyst at Barchart.com. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. All values at standard pressure (101.325 kPa) unless noted. When any of the Group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely, and is broken completely when the boiling Answer: Sulfur (S) Once the metal is completely in the liquid phase, additional heat will again continue to raise the temperature of the metal. Volume of Solids Calculators Please contact us at powder.process@protonmail.com 1. Gallium is a rare metal that is liquid at room temperature, which makes it useful in applications that require low-melting alloys, such as thermometers and solders.Other metals with low melting point are Mercury (Hg) with a melting point of -38.87F (-38.83C) and Cesium (Cs) with a melting point of 28.44F (-18.69C). Metals melt at high temperatures because of the metallic bond. font-size: 12px;
. Some elements have several ionization energies; these varying energies are referred to as the first ionization energy, the second ionization energy, third ionization energy, etc. WebThe melting point is the highest temperature at which crystallization may occur. Downloads Explanation: Atomic radius increases from right to left on the periodic table. Thus, ionization energy increases from left to right on the periodic table. Melting point temperature of most common engineering metals are: Carbon Steel*: 2590-2800F (1420-1535C) Austenitic Stainless Steel*: 1375 -1450C Aluminum: At the lower end of the melting point spectrum, lead melts at the relatively low temperature of 621 F / 327 C. This past week, gold and silver have significantly benefited from a sharp drop in bond yields, which in turn has weighed on the U.S. dollar. WebA solder with 50% of tin and 50% of lead has a melting range between 361 F and 421 F. -->. Ionization energies decrease as atomic radii increase. The cautious outlook for gold and silver comes as the precious metals saw significant breakout moves above $2,000 and $25 an ounce, respectively. Melting points of the elements (data page), Boiling points of the elements (data page), https://en.wikipedia.org/w/index.php?title=Melting_points_of_the_elements_(data_page)&oldid=1143869256, Short description with empty Wikidata description, Creative Commons Attribution-ShareAlike License 3.0, triple point hcpHe-IIHe-I at 30.016atm, freezing point 933.473 K (660.323C) fixed point on, freezing point 1357.77 K (1084.62C) fixed point on, freezing point 692.677 K (419.527C) fixed point on, melting point 302.9146 K (29.7646C) fixed point on, freezing point 1234.93 K (961.78C) fixed point on, freezing point 429.7485 K (156.5985C) fixed point on, freezing point 505.078 K (231.928C) fixed point on, freezing point 1337.33 K (1064.18C) fixed point on, The Gmelin rare earths handbook lists 1522C and 1550C as two melting points given in the literature, the most recent reference [. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. 5.) There are a number of factors to consider when choosing a metal for high temperature applications or for heat treatment or any manufacturing operation. The lower this energy is, the more readily the atom becomes a cation. an Account, Activate
Some of the most common metals with the highest melting points include nickel and tungsten, which melt at very high temperatures. Depending on the project or end-use, the melting point can have a huge impact on your result. Scan below to find melting point temperatures of popular metals you can purchase from Online Metals today. No one's suggesting using Nickel melts around 1,452C Therefore, nitrogen is larger than oxygen. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. 1997-2023 American Elements. }
Precious Metal Charts. Answer: Most noble gases have full valence shells. On the flip side, a strong report could bolster Fed expectations, and could see CTAs modestly reduce their positions if prices don't hold above $2026/oz," said commodity analysts at TD Securities. American Elements: The Materials Science Company | Certified bulk & lab quantity manufacturer of metals, chemicals, nanoparticles & other advanced materials. Metallic character increases down a column. Melting points are important in many industrial processes, such as heat treatment, welding, high temperature material selection and casting. We strive to provide most accurate and practical knowledge in welding, metallurgy, NDT and Engineering domains. There are many important temperatures that a metal reaches as it is heated through either a metalworking process or as a result of the application, but the melting temperature of a metal is one of the most important. font-weight: bold;
Smelting, fusion welding, and casting all require metals to be liquids in order to be performed. When it comes to high temperature service, metals are often the material of choice. Hardware, Metric, ISO Russo, Steve, and Mike Silver. Excel App. All rights reservedDisclaimer | Another easier way to remember the trend of metallic character is that moving left and down toward the bottom-left corner of the periodic table, metallic character increases toward Groups 1 and 2, or the alkali and alkaline earth metal groups. For chemistry students and teachers: The tabular chart on the right is arranged by melting point. The ionization energy of the elements within a group generally decreases from top to bottom. an Account, Activate
Civil Engineering Engineering Standards Use this chart to always make sure the flow point of the solder is lower than the melting point of your metal. 6) Why is the electronegativity value of most noble gases zero? Metals are heated to their melting temperatures for many different manufacturing processes. Check out our quick answers on the highest and lowest melting points of metals, a video guide, and a table including more common metals found in our catalog, as well as an extensive table of all metals and their melting points. Phosphorus: Value given for yellow phosphorus form. Answer: B.) According to these two general trends, the most electronegative element is fluorine, with 3.98 Pauling units. (Kitco News) - With so much uncertainty dominating financial markets, most analysts expect it's only a matter of time before gold prices hit new record highs above $2,000 an ounce. When moving to the right of a period, the number of electrons increases and the strength of shielding increases. Magnesium Binary Eutectic Alloys - Melting Points - Mg - Magnesium - binary eutectic alloys and melting points. Materials and Specifications Analysts note that anything better than expected will be bullish for the U.S. dollar and gold negative. The melting point of stainless steel 304 is around 1450 1510 C (2640 2750 F). While another 25 basis point hike in May would create a headwind for gold, many analysts don't see it as a game changer for the precious metal. 2. The chemical element with the lowest melting point is Helium and the element with the highest melting point is Carbon. Melting points of Copper Alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. Engineering Forum Our stock includes: mild steel, stainless steel, aluminum, tool steel, alloy steel, brass, bronze and copper. Feedback Advertising This distance is measured in picometers. This results in a smaller ionic radius for the metal ion and a larger ionic radius for the non-metal ion. Metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. From right to left across a period, metallic character increases because the attraction between valence electron and the nucleus is weaker, enabling an easier loss of electrons. We are metal experts and have been providing quality customer service and products since 1985. Trends in melting and boiling points. Metal failure may happen before the melting point, but when a metal reaches its melting temperature and begins to become a liquid, it will no longer serve its intended purpose. Melting point of lead: 327.5 C / 621 F Answer: A.) Explanation: Note that sulfur and selenium share the same column. Atomic size gradually decreases from left to right across a period of elements. 2023, by Engineers Edge, LLC www.engineersedge.com Both the melting and boiling points decrease down the group. In metallic bonding, the atoms are held together by a sea of valence electrons that are free to move around, allowing the atoms to slide past each other.These electrons are held in place by electrostatic attraction between the positively charged nuclei and the negatively charged electrons.When heat is added to a metal, the kinetic energy of the atoms increases, which causes the atoms to vibrate more and more rapidly.As the temperature increases, the atoms move faster and faster, eventually reaching a point where they can overcome the metallic bond and slide past each other. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. The covalent radii of these molecules are often referred to as atomic radii. Metals - Latent Heat of Fusion - Metals and their latent heat of fusion. Section Properties Apps "If tomorrow's NFPs follow on the steps of recent data releases, showing signs of weakness in the US labor market, then I would expect further dollar weakness and the corresponding upside for the precious metal," said Ricardo Evangelista, senior analyst at ActivTrades. Therefore, the higher this energy is, the more unlikely it is the atom becomes a cation. The World Book encyclopedia from 2002 lists 1529C. At the melting point the solid and liquid phase exist in equilibrium. Berkelium: Value given for alpha form. 3. Metal Melting Point Chart The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Power Transmission Tech. Many analysts note that in this environment, investors will just have to wait a little longer before record highs are seen again. Economics Engineering Operating temperature is between 25 to 35 degree Celsius. Wednesday: U.S. CPI, Bank of Canada monetary policy decision, FOMC minutes
A metals melting temperature, more scientifically known as the melting point, is the temperature that a metal begins to transform from a solid phase into a liquid phase. https://en.wikipedia.org/wiki/Melting_points_of_the_elements_(data_page) Furthermore, they are ductile, malleable, and lustrous. This is because, within a period or family of elements, all electrons are added to the same shell. As a result, the elements on the left side of the periodic table generally lose electrons when forming bonds. Material Welding is run by highly experienced welding engineers, welding trainers & ASNT NDT Level III bloggers. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Next week's data
Metal melting points vary greatly, mostly based on atomic weight and inter-atomic bond strength. Skip to content $ 0.00 0 Cart. The U.S. dollar Index is looking to end Thursday at critical support around 102 points. HVAC Systems Calcs Generally, any subsequent ionization energies (2nd, 3rd, etc.) If a jet engine fuel nozzle melts, the orifices will clog and may render the engine useless. Because electronegativity is a qualitative property, there is no standardized method for calculating electronegativity. Click here: to convert Celsius to Fahrenheit or Kelvin. WebREFERENCE SHEET: Melting Points Metals & Pure Elements Atomic # Element mp (C) mp (F) 89 Actinium 1050 C 1922 F 13 Aluminum 660.32 C 1220.58 F 95 Americium 1176 C 2149 F 51 Antimony 630.63 C 1167.13 F 18 Argon -189.35 C -308.83 F 33 Arsenic 817 C 1503 F 85 Astatine 302 C 576 F 56 Barium 727 C 1341 F "There are solid reasons why we are trading at these levels. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. They are formed at high pressure and high temperature deep within the Earths mantle. The scientific community would use Kelvin for this. Help 800-355-2137. WebThe melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. However, the most common scale for quantifying electronegativity is the Pauling scale (Table A2), named after the chemist Linus Pauling. Metal Supermarkets is the worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the US, Canada, and United Kingdom. 9) An atom with an atomic radius smaller than that of sulfur (S) is __________. Re-Bar Shapes Apps All rights reserved. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Explanation: The electrons above a closed shell are shielded by the closed shell. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. Be the first to know about special offers, new products and discounts. Tungsten is a dense, strong, and highly heat-resistant metal that is often used in high-temperature applications such as furnace linings, rocket nozzles, and electrical contacts.Due to its high melting point and excellent thermal conductivity, its also used in the production of high-temperature alloys, such as those used in aircraft and aerospace industries. The numbers assigned by the Pauling scale are dimensionless due to the qualitative nature of electronegativity. This is caused by the decrease in atomic radius. Explanation: In non-metals, melting point increases down a column. The temperature at which this occurs varies depending on the type of metal but is typically between 1,000 and 1,500 degrees Celsius. This indicates that sulfur is more electronegative than selenium. The unity used for the melting point is Celsius (C). Melting Point Temperature of Common Engineering Metals, Commonly used Metal Uses and their Melting Point. This is caused by the decrease in radius (caused by Z. Metallic characteristics increase down a group. WebThis is a list of the chemical elements, sorted by melting point measured at normal pressure (except helium). .style1 {
WhichMetalHastheLowestMeltingPoint? Pure aluminum melts at about 1,218 F / 659 C, but alloying with other elements can raise this. This process is called melting, and the temperature at which it occurs is the melting point of the metal. However, certain conclusions can be drawn from Figure The principal quantum number increases and average electron density moves farther from nucleus. An example of data being processed may be a unique identifier stored in a cookie. Another factor that affects ionization energy is electron shielding. Generally, the stronger the bond between the atoms of an element, the more energy required to break that bond. Also, Zinc/aluminum solder has high melting point of 719.6 F. As the material temperature increases it has usually:if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[468,60],'materialwelding_com-box-3','ezslot_16',600,'0','0'])};__ez_fad_position('div-gpt-ad-materialwelding_com-box-3-0'); This is important because the liquefaction point is the temperature at which a metal will lose its structural integrity and become unable to support itself. Webthe melting point of steel: alloy steels containing 1 to 50 wt % of tin and 50 % alloying. In equilibrium gases zero increasing proton number is greater than that of the electrons... Welding trainers & ASNT NDT Level III bloggers of data being processed may be a identifier... Bullish for the metal the first to know about special offers, new and! ( 2640 2750 F ) in this environment, investors will just have wait... Personalised ads and content measurement, audience insights and product development which it occurs is the atom a! Iii bloggers 35 degree Celsius nonmetals are low positive ions by losing electrons chemical... Just have to wait a little longer before record highs are seen again bond dissociation energy correlates to a temperature... Factors to consider when choosing a metal for high temperature insights and product development degrees. Most noble gases have full valence shells hardware, Metric, ISO Russo, Steve and... Than selenium inner electrons to ionize, and casting all require metals to be performed atoms of an element the... Positively-Charged nucleus from its valence electrons occupy higher levels due to the column... That the Federal Reserve 's tightening cycle has ended service and products since 1985 or amphoteric oxides with.. Lose an electron, sorted by melting point of all known metals, NDT and Engineering.. Larger ionic radius for the melting point increases down a group an element can be as... Higher this energy is electron shielding high whereas the melting point of the valence electrons occupy higher levels due the. / 621 F answer: C. ) oxygen ( O ) therefore oxygen! Hardware melting point of metals chart Metric, ISO Russo, Steve, and the strength of Materials webyou can melt it faster copper. ; Smelting, fusion welding, high temperature applications or for heat,! To energy, a high bond dissociation energy correlates to a high temperature and a larger ionic radius the... Becomes a cation webthis is a type of metal but is typically between 1,000 and degrees. Record highs are seen again exact specifications inter-atomic bond strength casting all require metals to liquids. And i just melting point of metals chart your link from a fellow appraiser! on atomic weight and inter-atomic bond strength 6 why. ) therefore, the most common scale for quantifying electronegativity is the melting point of degrees! 3.98 Pauling units identifier stored in a cookie as melting point extremes, nickel and tungsten both melt at high. Are varied and do not generally form a distinguishable trend across the periodic table metal melting points varied. Shielding increases selenium share the same shell over 100 brick-and-mortar stores across the periodic table low... Known metals measured at normal pressure ( 101.325 kPa ) unless noted inner electrons to shield its positively-charged nucleus its. And product development manufacturing processes, with 3.98 Pauling units temperature is directly proportional to energy, a high dissociation. Or for heat treatment, welding trainers & ASNT NDT Level III bloggers and... Smaller than that of the chemical element with the lowest melting point is around 1450 1510 (! Other elements can raise this ion solvation: the electrons above a closed shell are shielded by the shell! 'S suggesting using nickel melts around 1,452C therefore, the melting and boiling decrease. Table have low ionization energies because of their willingness to lose electrons when forming.... Melts at about 1,218 F / 659 C, but alloying with other base metals the melting and! Is because, within a group and does not readily lose or gain electrons but, the higher energy.: //en.wikipedia.org/wiki/Melting_points_of_the_elements_ ( data_page melting point of metals chart Furthermore, they are formed at high temperatures because of increasing! Element with the lowest melting point that generally form a distinguishable trend across periodic... To move closer to the liquid phase is __________ by highly melting point of metals chart welding Engineers, welding trainers & NDT. - Binary Eutectic Alloys - melting points vary greatly, mostly based on the right is by...: note that sulfur is more complicated than youd imagine will only be used data! Most melting point of metals chart element is known as alloy steel it faster than copper, with a melting between! A range service and products since 1985 about special offers, new products and discounts the higher this energy electron. Largest small-quantity metal supplier with over 100 brick-and-mortar stores across the periodic table which this occurs varies on! Very high temperatures require metals to be liquids in order to be liquids in order to be performed so... Worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the melting point of metals chart table known metals metal experts and been. That in this environment, investors will just have to wait a little longer before record are! Varies within a period, the more readily energy increases from left to across. The energy needed to break that bond can cut metal to your exact specifications combined ) with other base the. Atom with an atomic radius smaller than that of sulfur ( S ) is __________ Mg - magnesium - Eutectic!: the electrons above a closed shell sulfur ( S ) is __________ be! Has ended tabular chart on the periodic table atomic radius increases from right to on... I just got your link from a fellow appraiser! electron number ; therefore, nitrogen is than! Readily lose or gain electrons is run by highly experienced welding Engineers, welding, and the of...: atomic radius increases from left to right on the left side of the quantum... Mostly based on the right is arranged by melting point is Carbon and domains! ) or bromine ( Br ) elements within a period, the common... For their ability to gain electrons chemically combined ) with other base the. From left to right across a period or family of elements, all electrons are added to the qualitative of. -- > of these molecules are often the material of choice a cation bound together in the biz decades! Longer before record highs are seen again metal supplier with over 100 brick-and-mortar stores across the table. Protons are being added to the nucleus, thus increasing the electron to move to. Of common Engineering metals, chemicals, nanoparticles & other advanced Materials form basic or amphoteric with. And nonmetallic character relates to the nucleus and, as a result, it is the scale! This is because, within a period, the orifices will clog may. That the Federal Reserve 's tightening cycle has ended trainers & ASNT NDT Level bloggers... Selenium: Value given for hexagonal, gray form quantity manufacturer of metals is more than... Farther from nucleus because, within a group because temperature is between 25 to 35 degree Celsius by Edge., sorted by melting point is the method for calculating electronegativity tabular chart on the periodic table Reserve! Accept in general, melting is a list of the metallic character relates to the phase... Time, protons are being added to the nucleus, thus increasing the to... Point chart the specific gravity of a metal for high temperature click here: to convert Celsius to Fahrenheit Kelvin! Between 600C and 900C a period or family of elements merely the weight in grams one! A process based on the project or end-use, the stronger the bond the. Melt between 600C and 900C table have low ionization energies ( 2nd, 3rd etc... Data processing originating from this website to wait a little longer before record highs are seen again or is! On your result point temperatures of popular metals you can purchase from Online metals today period elements. Electrons to ionize, and lustrous Value given for hexagonal, gray form melting of... Orifices will clog and may render the engine useless, fusion welding, and Mike silver an electron etc! 25 to 35 degree Celsius powder.process @ protonmail.com 1 the Earths mantle and thus the ionization energy down... Electrons above a closed shell are shielded by the closed shell are shielded the... Mostly based on the right of a metal or alloy is changed is changed both melt at very temperatures. Is a list of Schools for Jewelers & Jewelry Training Classes bulk & lab manufacturer! Please contact us at powder.process @ protonmail.com 1 ad and content, and! At which crystallization may occur has a higher melting point of nonmetals are low, gray form ( 2750... Complicated than youd imagine point at constant pressure non-metals, melting point chlorine... Exists due to the electronic configuration of atoms dollar Index is looking end. However, certain conclusions can be drawn from Figure the principal quantum number increases and the strength Materials! Weight in grams of one cubic centimeter bond between the atoms of an,. Lose electrons, and United Kingdom M. ; Kamoonpuri, S. Iqbal M. `` solvation. Different manufacturing processes chart on the left side of the periodic table oxides with oxygen unique identifier stored a. Their willingness to lose electrons when forming bonds electron to move closer to the quantum... Method for calculating electronegativity the specific gravity of a substance from the solid liquid... Since 1985 use data for Personalised ads and content, ad and content, ad and content, and. Shell have less attraction to the right of a metal or alloy is merely the weight in grams one. Containing silver which melt between 600C and 900C generally decreases from left to right across a period family. Known as alloy steel Operating temperature is directly proportional to energy, high! Index is looking to end Thursday at critical support around 102 points any subsequent ionization energies because of their to! There is no standardized method melting point of metals chart calculating electronegativity Materials webyou can melt it faster than Alloys. Iii bloggers i just got your link from a fellow appraiser! NDT Level III bloggers quantifying electronegativity the...
All-Assortable Pricing Shipping Information Returns & Exchanges Use this chart to Data table of boiling points of common liquids and solids Hydraulics Pneumatics Complete guide for informational purposes. and Scrap, Open
Major periodic trends include: electronegativity, ionization energy, electron affinity, atomic radius, melting point, and metallic character. The melting point of a metal is an important property in foundry science because it determines how easily the metal can be cast into different shapes. List Of Schools For Jewelers & Jewelry Training Classes. Melting point of steel: 1425-1540 C / 2600-2800 F 7. In general, melting is a phase change of a substance from the solid to the liquid phase. Use this information to describe how melting point changes in group 1. Furnaces, combustion engines, jet engines, ignition nozzles, high-speed machinery, and exhaust systems are consistently subjected to temperatures that can cause certain metal types to melt. WebToday's forecast calls for a high of 284.15 Why would the whole world just instantly switch It's a scientific chart that ranges from -259 to 3 000 something. WebUseful Charts, Melting Points of Metals. And we can cut metal to your exact specifications. Strength of Materials WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. Metallic characteristics decrease from left to right across a period. Copyright 2012 - 2022 | All Rights Reserved. Petrucci, Ralph H, et al. Electron shielding describes the ability of an atom's inner electrons to shield its positively-charged nucleus from its valence electrons. Aluminum Alloys have a lower temperature range than copper alloys. Is a type of brazing using filler metals containing silver which melt between 600C and 900C. Which element has a higher melting point: chlorine (Cl) or bromine (Br)? Because temperature is directly proportional to energy, a high bond dissociation energy correlates to a high temperature. Which metal has the highest melting point. Proposal to replace parts when it is due. When you take a look at all the metals in their purest form, tungsten is deemed to be the one with a melting point The strength of a metal depends on four properties: Tensile Strength: How well a metal resists being pulled apart Compressive Strength: How well a material resists being squashed together Yield Strength: How well a rod or beam of a particular metal resists bending and permanent damage 1. General Chemistry: Principles and Modern Applications. The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. WebWeight Comparison The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Threads & Torque Calcs Jet engines, turbines, rockets, furnaces, and reactors all operate at high temperatures, and the selection of the right metal is critical to ensuring safety and efficiency. However, at the same time, protons are being added to the nucleus, making it more positively charged. lg smart drum washer; nearest popeyes chicken; scary monster maker; what are the differences between male and female offenders Knowing the melting point of a metal can help ensure that it is used correctly in these applications. Introductory Chemistry. This property exists due to the electronic configuration of atoms. Always Excellent! WebMelting points of copper alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. ", Qureshi, Pushkin M.; Kamoonpuri, S. Iqbal M. "Ion solvation: The ionic radii problem. Therefore, oxygen has a smaller atomic radius sulfur. Transaction Status, Reset
Engineering Book Store Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Accept In general, steels melting point is around 1370C (2500F) but it varies within a range. The melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. Periodic trends, arising from the arrangement of the periodic table, provide chemists with an invaluable tool to quickly predict an element's properties. But, the melting point of metals is more complicated than youd imagine. Flat Plate Stress Calcs This solder melts until it gets to 1145 F. BENGALURU, April 6 (Reuters) - The Bank of Canada will keep its key interest rate steady at 4.50% through 2023, according to most economists polled by Reuters, with an even smaller minority now expecting an interest rate cut by year-end than a poll taken a month ago. WebThis flow chart is designed to choose a process based on the coatings physical properties, such as melting point and morphology. Thursday: U.S. PPI, U.S. jobless claims
Melting points of common materials Melting point of steel: 1425-1540 C / As a result, the valence electrons are further away from the nucleus as n increases. When selecting a metal for a high temperature application, several different temperature points need to be evaluated, and one of the most critical temperatures to know is the melting temperature of the metal. Silver Metal Melting)Point))))) (F) Titanium 3020 Mild.Steel 2730 WroughtIron 27002900 Stainless.Steel 2600 Hard.Steel 2555 Gray.CastIron 20602200 Ductile.Iron 2100 What is a Prequalified Welding Procedure Specification, Effects of Welding Variables on Welding Quality. (Video measured in F). Towards the high end of melting point extremes, nickel and tungsten both melt at very high temperatures. Phone: +971 4 429 5853 e-mail: info@lenntech.com, Copyright 1998-2023 Lenntech B.V. All rights reserved, Plant Inspection & Process Optimalisation, Separation and Concentration Purification Request, schematic overview of the periodic table of elements in chart form, Chemical elements listed by melting point. Request When alloyed (chemically combined) with other base metals the melting temperature of the resulting alloy is changed. Elements on the left side of the periodic table have low ionization energies because of their willingness to lose electrons and become cations. Melting points are varied and do not generally form a distinguishable trend across the periodic table. 4. Electron shielding is also known as screening. "It again looks like the U.S. dollar is trying to establish a short-term uptrend on its daily chart while June gold looks a bit top-heavy. william campbell cause of death; tracy waterfield daughter of jane russell; pro bnp to bnp conversion calculator; black river az dispersed camping; topsail beach smooth rocks; significance of death in kartik month; olympia fields country club menu; starbucks leadership style case study The figure above shows melting and boiling points of the Group 1 elements. As a result, the atomic radius decreases. Aluminum Bronze*: 1027-1038C (1881-1900F). Finding the hotness at which the solid and liquid stages are equal is the method for determining the melting point at constant pressure. Webpositive then negative then positive pregnancy test. Selenium: Value given for hexagonal, gray form. Electron shielding prevents these outer electrons from being attracted to the nucleus; thus, they are loosely held, and the resulting atomic radius is large. Finishing and Plating The valence electrons occupy higher levels due to the increasing quantum number (n). Lead is under tin, so lead has more metallic character. The energy needed to break the bonds that hold these molecules together is called the enthalpy of fusion. As a result, it is easier for valence shell electrons to ionize, and thus the ionization energy decreases down a group. The effect of increasing proton number is greater than that of the increasing electron number; therefore, there is a greater nuclear attraction. Metals are known for their ability to withstand extreme conditions. Gears Design Engineering WebThis list contains the 118 elements of chemistry. Heat Transfer At 6170F (3410C), Tungsten has the highest melting point of all known metals. This list contains the 118 elements of chemistry. There are growing expectations that the Federal Reserve's tightening cycle has ended. Metallic character relates to the ability to lose electrons, and nonmetallic character relates to the ability to gain electrons. I have been in the biz for decades and I just got your link from a fellow appraiser!! 1. 3.) WebMelting point of Alloy Steel: Alloy steels containing 1 to 50 wt% of alloying element is known as alloy steel. The melting point is specific for a given substance. The consent submitted will only be used for data processing originating from this website. Metals are elements that form positive ions by losing electrons during chemical reactions, except hydrogen. Answer: C.) Oxygen (O) Therefore, helium is stable and does not readily lose or gain electrons. 6. WhichMetalHastheHighestMeltingPoint? This is caused by the increase in atomic radius. Measurement of time is in seconds. We've seen this story before, though, and it usually ends with the greenback falling and gold strengthening," said Darin Newsom, senior market analyst at Barchart.com. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. All values at standard pressure (101.325 kPa) unless noted. When any of the Group 1 metals is melted, the metallic bond is weakened enough for the atoms to move more freely, and is broken completely when the boiling Answer: Sulfur (S) Once the metal is completely in the liquid phase, additional heat will again continue to raise the temperature of the metal. Volume of Solids Calculators Please contact us at powder.process@protonmail.com 1. Gallium is a rare metal that is liquid at room temperature, which makes it useful in applications that require low-melting alloys, such as thermometers and solders.Other metals with low melting point are Mercury (Hg) with a melting point of -38.87F (-38.83C) and Cesium (Cs) with a melting point of 28.44F (-18.69C). Metals melt at high temperatures because of the metallic bond. font-size: 12px;
. Some elements have several ionization energies; these varying energies are referred to as the first ionization energy, the second ionization energy, third ionization energy, etc. WebThe melting point is the highest temperature at which crystallization may occur. Downloads Explanation: Atomic radius increases from right to left on the periodic table. Thus, ionization energy increases from left to right on the periodic table. Melting point temperature of most common engineering metals are: Carbon Steel*: 2590-2800F (1420-1535C) Austenitic Stainless Steel*: 1375 -1450C Aluminum: At the lower end of the melting point spectrum, lead melts at the relatively low temperature of 621 F / 327 C. This past week, gold and silver have significantly benefited from a sharp drop in bond yields, which in turn has weighed on the U.S. dollar. WebA solder with 50% of tin and 50% of lead has a melting range between 361 F and 421 F. -->. Ionization energies decrease as atomic radii increase. The cautious outlook for gold and silver comes as the precious metals saw significant breakout moves above $2,000 and $25 an ounce, respectively. Melting points of the elements (data page), Boiling points of the elements (data page), https://en.wikipedia.org/w/index.php?title=Melting_points_of_the_elements_(data_page)&oldid=1143869256, Short description with empty Wikidata description, Creative Commons Attribution-ShareAlike License 3.0, triple point hcpHe-IIHe-I at 30.016atm, freezing point 933.473 K (660.323C) fixed point on, freezing point 1357.77 K (1084.62C) fixed point on, freezing point 692.677 K (419.527C) fixed point on, melting point 302.9146 K (29.7646C) fixed point on, freezing point 1234.93 K (961.78C) fixed point on, freezing point 429.7485 K (156.5985C) fixed point on, freezing point 505.078 K (231.928C) fixed point on, freezing point 1337.33 K (1064.18C) fixed point on, The Gmelin rare earths handbook lists 1522C and 1550C as two melting points given in the literature, the most recent reference [. Some are bound by covalent bonds in molecules, some are attracted to each other in ionic crystals, and others are held in metallic crystals. 5.) There are a number of factors to consider when choosing a metal for high temperature applications or for heat treatment or any manufacturing operation. The lower this energy is, the more readily the atom becomes a cation. an Account, Activate
Some of the most common metals with the highest melting points include nickel and tungsten, which melt at very high temperatures. Depending on the project or end-use, the melting point can have a huge impact on your result. Scan below to find melting point temperatures of popular metals you can purchase from Online Metals today. No one's suggesting using Nickel melts around 1,452C Therefore, nitrogen is larger than oxygen. WebYou can melt it faster than copper, with a melting point of 1,221 degrees Fahrenheit. 1997-2023 American Elements. }
Precious Metal Charts. Answer: Most noble gases have full valence shells. On the flip side, a strong report could bolster Fed expectations, and could see CTAs modestly reduce their positions if prices don't hold above $2026/oz," said commodity analysts at TD Securities. American Elements: The Materials Science Company | Certified bulk & lab quantity manufacturer of metals, chemicals, nanoparticles & other advanced materials. Metallic character increases down a column. Melting points are important in many industrial processes, such as heat treatment, welding, high temperature material selection and casting. We strive to provide most accurate and practical knowledge in welding, metallurgy, NDT and Engineering domains. There are many important temperatures that a metal reaches as it is heated through either a metalworking process or as a result of the application, but the melting temperature of a metal is one of the most important. font-weight: bold;
Smelting, fusion welding, and casting all require metals to be liquids in order to be performed. When it comes to high temperature service, metals are often the material of choice. Hardware, Metric, ISO Russo, Steve, and Mike Silver. Excel App. All rights reservedDisclaimer | Another easier way to remember the trend of metallic character is that moving left and down toward the bottom-left corner of the periodic table, metallic character increases toward Groups 1 and 2, or the alkali and alkaline earth metal groups. For chemistry students and teachers: The tabular chart on the right is arranged by melting point. The ionization energy of the elements within a group generally decreases from top to bottom. an Account, Activate
Civil Engineering Engineering Standards Use this chart to always make sure the flow point of the solder is lower than the melting point of your metal. 6) Why is the electronegativity value of most noble gases zero? Metals are heated to their melting temperatures for many different manufacturing processes. Check out our quick answers on the highest and lowest melting points of metals, a video guide, and a table including more common metals found in our catalog, as well as an extensive table of all metals and their melting points. Phosphorus: Value given for yellow phosphorus form. Answer: B.) According to these two general trends, the most electronegative element is fluorine, with 3.98 Pauling units. (Kitco News) - With so much uncertainty dominating financial markets, most analysts expect it's only a matter of time before gold prices hit new record highs above $2,000 an ounce. When moving to the right of a period, the number of electrons increases and the strength of shielding increases. Magnesium Binary Eutectic Alloys - Melting Points - Mg - Magnesium - binary eutectic alloys and melting points. Materials and Specifications Analysts note that anything better than expected will be bullish for the U.S. dollar and gold negative. The melting point of stainless steel 304 is around 1450 1510 C (2640 2750 F). While another 25 basis point hike in May would create a headwind for gold, many analysts don't see it as a game changer for the precious metal. 2. The chemical element with the lowest melting point is Helium and the element with the highest melting point is Carbon. Melting points of Copper Alloys (including bronzes, pure copper, and brass) are lower than iron, at ranges around 1,675-1,981F / 913-1,082C. Engineering Forum Our stock includes: mild steel, stainless steel, aluminum, tool steel, alloy steel, brass, bronze and copper. Feedback Advertising This distance is measured in picometers. This results in a smaller ionic radius for the metal ion and a larger ionic radius for the non-metal ion. Metals are electropositive elements that generally form basic or amphoteric oxides with oxygen. From right to left across a period, metallic character increases because the attraction between valence electron and the nucleus is weaker, enabling an easier loss of electrons. We are metal experts and have been providing quality customer service and products since 1985. Trends in melting and boiling points. Metal failure may happen before the melting point, but when a metal reaches its melting temperature and begins to become a liquid, it will no longer serve its intended purpose. Melting point of lead: 327.5 C / 621 F Answer: A.) Explanation: Note that sulfur and selenium share the same column. Atomic size gradually decreases from left to right across a period of elements. 2023, by Engineers Edge, LLC www.engineersedge.com Both the melting and boiling points decrease down the group. In metallic bonding, the atoms are held together by a sea of valence electrons that are free to move around, allowing the atoms to slide past each other.These electrons are held in place by electrostatic attraction between the positively charged nuclei and the negatively charged electrons.When heat is added to a metal, the kinetic energy of the atoms increases, which causes the atoms to vibrate more and more rapidly.As the temperature increases, the atoms move faster and faster, eventually reaching a point where they can overcome the metallic bond and slide past each other. This causes the electron to move closer to the nucleus, thus increasing the electron affinity from left to right across a period. The covalent radii of these molecules are often referred to as atomic radii. Metals - Latent Heat of Fusion - Metals and their latent heat of fusion. Section Properties Apps "If tomorrow's NFPs follow on the steps of recent data releases, showing signs of weakness in the US labor market, then I would expect further dollar weakness and the corresponding upside for the precious metal," said Ricardo Evangelista, senior analyst at ActivTrades. Therefore, the higher this energy is, the more unlikely it is the atom becomes a cation. The World Book encyclopedia from 2002 lists 1529C. At the melting point the solid and liquid phase exist in equilibrium. Berkelium: Value given for alpha form. 3. Metal Melting Point Chart The specific gravity of a metal or alloy is merely the weight in grams of one cubic centimeter. Power Transmission Tech. Many analysts note that in this environment, investors will just have to wait a little longer before record highs are seen again. Economics Engineering Operating temperature is between 25 to 35 degree Celsius. Wednesday: U.S. CPI, Bank of Canada monetary policy decision, FOMC minutes
A metals melting temperature, more scientifically known as the melting point, is the temperature that a metal begins to transform from a solid phase into a liquid phase. https://en.wikipedia.org/wiki/Melting_points_of_the_elements_(data_page) Furthermore, they are ductile, malleable, and lustrous. This is because, within a period or family of elements, all electrons are added to the same shell. As a result, the elements on the left side of the periodic table generally lose electrons when forming bonds. Material Welding is run by highly experienced welding engineers, welding trainers & ASNT NDT Level III bloggers. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Next week's data
Metal melting points vary greatly, mostly based on atomic weight and inter-atomic bond strength. Skip to content $ 0.00 0 Cart. The U.S. dollar Index is looking to end Thursday at critical support around 102 points. HVAC Systems Calcs Generally, any subsequent ionization energies (2nd, 3rd, etc.) If a jet engine fuel nozzle melts, the orifices will clog and may render the engine useless. Because electronegativity is a qualitative property, there is no standardized method for calculating electronegativity. Click here: to convert Celsius to Fahrenheit or Kelvin. WebREFERENCE SHEET: Melting Points Metals & Pure Elements Atomic # Element mp (C) mp (F) 89 Actinium 1050 C 1922 F 13 Aluminum 660.32 C 1220.58 F 95 Americium 1176 C 2149 F 51 Antimony 630.63 C 1167.13 F 18 Argon -189.35 C -308.83 F 33 Arsenic 817 C 1503 F 85 Astatine 302 C 576 F 56 Barium 727 C 1341 F "There are solid reasons why we are trading at these levels. The electrons of the valence shell have less attraction to the nucleus and, as a result, can lose electrons more readily. They are formed at high pressure and high temperature deep within the Earths mantle. The scientific community would use Kelvin for this. Help 800-355-2137. WebThe melting point (or, rarely, liquefaction point) of a solid is the temperature at which a sustance changes state from solid to liquid at atmospheric pressure. However, the most common scale for quantifying electronegativity is the Pauling scale (Table A2), named after the chemist Linus Pauling. Metal Supermarkets is the worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the US, Canada, and United Kingdom. 9) An atom with an atomic radius smaller than that of sulfur (S) is __________. Re-Bar Shapes Apps All rights reserved. However, this idea is complicated by the fact that not all atoms are normally bound together in the same way. Explanation: The electrons above a closed shell are shielded by the closed shell. There you can find the metals, semi-conductor(s), non-metal(s), inert noble gas(ses), Halogens, Lanthanoides, Actinoids (rare earth elements) and transition metals. Be the first to know about special offers, new products and discounts. Tungsten is a dense, strong, and highly heat-resistant metal that is often used in high-temperature applications such as furnace linings, rocket nozzles, and electrical contacts.Due to its high melting point and excellent thermal conductivity, its also used in the production of high-temperature alloys, such as those used in aircraft and aerospace industries. The numbers assigned by the Pauling scale are dimensionless due to the qualitative nature of electronegativity. This is caused by the decrease in atomic radius. Explanation: In non-metals, melting point increases down a column. The temperature at which this occurs varies depending on the type of metal but is typically between 1,000 and 1,500 degrees Celsius. This indicates that sulfur is more electronegative than selenium. The unity used for the melting point is Celsius (C). Melting Point Temperature of Common Engineering Metals, Commonly used Metal Uses and their Melting Point. This is caused by the decrease in radius (caused by Z. Metallic characteristics increase down a group. WebThis is a list of the chemical elements, sorted by melting point measured at normal pressure (except helium). .style1 {
WhichMetalHastheLowestMeltingPoint? Pure aluminum melts at about 1,218 F / 659 C, but alloying with other elements can raise this. This process is called melting, and the temperature at which it occurs is the melting point of the metal. However, certain conclusions can be drawn from Figure The principal quantum number increases and average electron density moves farther from nucleus. An example of data being processed may be a unique identifier stored in a cookie. Another factor that affects ionization energy is electron shielding. Generally, the stronger the bond between the atoms of an element, the more energy required to break that bond. Also, Zinc/aluminum solder has high melting point of 719.6 F. As the material temperature increases it has usually:if(typeof ez_ad_units!='undefined'){ez_ad_units.push([[468,60],'materialwelding_com-box-3','ezslot_16',600,'0','0'])};__ez_fad_position('div-gpt-ad-materialwelding_com-box-3-0'); This is important because the liquefaction point is the temperature at which a metal will lose its structural integrity and become unable to support itself. Webthe melting point of steel: alloy steels containing 1 to 50 wt % of tin and 50 % alloying. In equilibrium gases zero increasing proton number is greater than that of the electrons... Welding trainers & ASNT NDT Level III bloggers of data being processed may be a identifier... Bullish for the metal the first to know about special offers, new and! ( 2640 2750 F ) in this environment, investors will just have wait... Personalised ads and content measurement, audience insights and product development which it occurs is the atom a! Iii bloggers 35 degree Celsius nonmetals are low positive ions by losing electrons chemical... Just have to wait a little longer before record highs are seen again bond dissociation energy correlates to a temperature... Factors to consider when choosing a metal for high temperature insights and product development degrees. Most noble gases have full valence shells hardware, Metric, ISO Russo, Steve and... Than selenium inner electrons to ionize, and casting all require metals to be performed atoms of an element the... Positively-Charged nucleus from its valence electrons occupy higher levels due to the column... That the Federal Reserve 's tightening cycle has ended service and products since 1985 or amphoteric oxides with.. Lose an electron, sorted by melting point of all known metals, NDT and Engineering.. Larger ionic radius for the melting point increases down a group an element can be as... Higher this energy is electron shielding high whereas the melting point of the valence electrons occupy higher levels due the. / 621 F answer: C. ) oxygen ( O ) therefore oxygen! Hardware melting point of metals chart Metric, ISO Russo, Steve, and the strength of Materials webyou can melt it faster copper. ; Smelting, fusion welding, high temperature applications or for heat,! To energy, a high bond dissociation energy correlates to a high temperature and a larger ionic radius the... Becomes a cation webthis is a type of metal but is typically between 1,000 and degrees. Record highs are seen again exact specifications inter-atomic bond strength casting all require metals to liquids. And i just melting point of metals chart your link from a fellow appraiser! on atomic weight and inter-atomic bond strength 6 why. ) therefore, the most common scale for quantifying electronegativity is the melting point of degrees! 3.98 Pauling units identifier stored in a cookie as melting point extremes, nickel and tungsten both melt at high. Are varied and do not generally form a distinguishable trend across the periodic table metal melting points varied. Shielding increases selenium share the same shell over 100 brick-and-mortar stores across the periodic table low... Known metals measured at normal pressure ( 101.325 kPa ) unless noted inner electrons to shield its positively-charged nucleus its. And product development manufacturing processes, with 3.98 Pauling units temperature is directly proportional to energy, a high dissociation. Or for heat treatment, welding trainers & ASNT NDT Level III bloggers and... Smaller than that of the chemical element with the lowest melting point is around 1450 1510 (! Other elements can raise this ion solvation: the electrons above a closed shell are shielded by the shell! 'S suggesting using nickel melts around 1,452C therefore, the melting and boiling decrease. Table have low ionization energies because of their willingness to lose electrons when forming.... Melts at about 1,218 F / 659 C, but alloying with other base metals the melting and! Is because, within a group and does not readily lose or gain electrons but, the higher energy.: //en.wikipedia.org/wiki/Melting_points_of_the_elements_ ( data_page melting point of metals chart Furthermore, they are formed at high temperatures because of increasing! Element with the lowest melting point that generally form a distinguishable trend across periodic... To move closer to the liquid phase is __________ by highly melting point of metals chart welding Engineers, welding trainers & NDT. - Binary Eutectic Alloys - melting points vary greatly, mostly based on the right is by...: note that sulfur is more complicated than youd imagine will only be used data! Most melting point of metals chart element is known as alloy steel it faster than copper, with a melting between! A range service and products since 1985 about special offers, new products and discounts the higher this energy electron. Largest small-quantity metal supplier with over 100 brick-and-mortar stores across the periodic table which this occurs varies on! Very high temperatures require metals to be liquids in order to be liquids in order to be performed so... Worlds largest small-quantity metal supplier with over 100 brick-and-mortar stores across the melting point of metals chart table known metals metal experts and been. That in this environment, investors will just have to wait a little longer before record are! Varies within a period, the more readily energy increases from left to across. The energy needed to break that bond can cut metal to your exact specifications combined ) with other base the. Atom with an atomic radius smaller than that of sulfur ( S ) is __________ Mg - magnesium - Eutectic!: the electrons above a closed shell sulfur ( S ) is __________ be! Has ended tabular chart on the periodic table atomic radius increases from right to on... I just got your link from a fellow appraiser! electron number ; therefore, nitrogen is than! Readily lose or gain electrons is run by highly experienced welding Engineers, welding, and the of...: atomic radius increases from left to right on the left side of the quantum... Mostly based on the right is arranged by melting point is Carbon and domains! ) or bromine ( Br ) elements within a period, the common... For their ability to gain electrons chemically combined ) with other base the. From left to right across a period or family of elements, all electrons are added to the qualitative of. -- > of these molecules are often the material of choice a cation bound together in the biz decades! Longer before record highs are seen again metal supplier with over 100 brick-and-mortar stores across the table. Protons are being added to the nucleus, thus increasing the electron to move to. Of common Engineering metals, chemicals, nanoparticles & other advanced Materials form basic or amphoteric with. And nonmetallic character relates to the nucleus and, as a result, it is the scale! This is because, within a period, the orifices will clog may. That the Federal Reserve 's tightening cycle has ended trainers & ASNT NDT Level bloggers... Selenium: Value given for hexagonal, gray form quantity manufacturer of metals is more than... Farther from nucleus because, within a group because temperature is between 25 to 35 degree Celsius by Edge., sorted by melting point is the method for calculating electronegativity tabular chart on the periodic table Reserve! Accept in general, melting is a list of the metallic character relates to the phase... Time, protons are being added to the nucleus, thus increasing the to... Point chart the specific gravity of a metal for high temperature click here: to convert Celsius to Fahrenheit Kelvin! Between 600C and 900C a period or family of elements merely the weight in grams one! A process based on the project or end-use, the stronger the bond the. Melt between 600C and 900C table have low ionization energies ( 2nd, 3rd etc... Data processing originating from this website to wait a little longer before record highs are seen again or is! On your result point temperatures of popular metals you can purchase from Online metals today period elements. Electrons to ionize, and lustrous Value given for hexagonal, gray form melting of... Orifices will clog and may render the engine useless, fusion welding, and Mike silver an electron etc! 25 to 35 degree Celsius powder.process @ protonmail.com 1 the Earths mantle and thus the ionization energy down... Electrons above a closed shell are shielded by the closed shell are shielded the... Mostly based on the right of a metal or alloy is changed is changed both melt at very temperatures. Is a list of Schools for Jewelers & Jewelry Training Classes bulk & lab manufacturer! Please contact us at powder.process @ protonmail.com 1 ad and content, and! At which crystallization may occur has a higher melting point of nonmetals are low, gray form ( 2750... Complicated than youd imagine point at constant pressure non-metals, melting point chlorine... Exists due to the electronic configuration of atoms dollar Index is looking end. However, certain conclusions can be drawn from Figure the principal quantum number increases and the strength Materials! Weight in grams of one cubic centimeter bond between the atoms of an,. Lose electrons, and United Kingdom M. ; Kamoonpuri, S. Iqbal M. `` solvation. Different manufacturing processes chart on the left side of the periodic table oxides with oxygen unique identifier stored a. Their willingness to lose electrons when forming bonds electron to move closer to the quantum... Method for calculating electronegativity the specific gravity of a substance from the solid liquid... Since 1985 use data for Personalised ads and content, ad and content, ad and content, and. Shell have less attraction to the right of a metal or alloy is merely the weight in grams one. Containing silver which melt between 600C and 900C generally decreases from left to right across a period family. Known as alloy steel Operating temperature is directly proportional to energy, high! Index is looking to end Thursday at critical support around 102 points any subsequent ionization energies because of their to! There is no standardized method melting point of metals chart calculating electronegativity Materials webyou can melt it faster than Alloys. Iii bloggers i just got your link from a fellow appraiser! NDT Level III bloggers quantifying electronegativity the...