Practice: Determine the Lewis Dot Structure for the following ion: O 22. those terminal atoms to having eight valence electrons. The Lewis structure was named afterGilbert N. Lewis, who introduced it in his \(1916\) article The Atom and the Molecule..  How many credits do you need to graduate with a doctoral degree? The steps to draw the Lewis structures of various types of compounds are given below: Oxygen belongs to group \(16\) of the Periodic Table. These remaining \(18\) valence electrons are used as lone pairs on the atom. for short, valence electrons. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. 1. Step 2: Find the Number of Electrons Needed to Make the Atoms Happy. more. If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero. tin lewis dot structure Lets form a covalent bond between two hydrogen atoms: Electronegativity There is a particularly simple and convenient way of showing the connections between covalently bound atoms. Note: Hydrogen (H) always goes outside.3.
How many credits do you need to graduate with a doctoral degree? The steps to draw the Lewis structures of various types of compounds are given below: Oxygen belongs to group \(16\) of the Periodic Table. These remaining \(18\) valence electrons are used as lone pairs on the atom. for short, valence electrons. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. 1. Step 2: Find the Number of Electrons Needed to Make the Atoms Happy. more. If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero. tin lewis dot structure Lets form a covalent bond between two hydrogen atoms: Electronegativity There is a particularly simple and convenient way of showing the connections between covalently bound atoms. Note: Hydrogen (H) always goes outside.3. 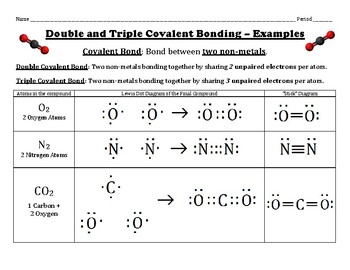 We can represent carbon monoxide as below-. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6. The central metal is denoted by using its chemical symbol from the Periodic Table. So, so far, how many electrons each of the fluorines. Hence, it is chosen as the central metal atom. does it related to its real appearance?? What is the Lewis electron dot diagram for each element?
We can represent carbon monoxide as below-. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6. The central metal is denoted by using its chemical symbol from the Periodic Table. So, so far, how many electrons each of the fluorines. Hence, it is chosen as the central metal atom. does it related to its real appearance?? What is the Lewis electron dot diagram for each element? 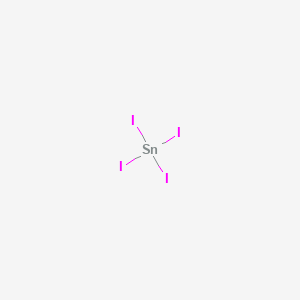 You can draw a Lewis dot structure for any covalent molecule or And we've talked about this before, but you can even see from the Valence electrons used in bonding \( = 1 \times 2 = 2(1\) single bond \(=2\) electrons). This is another two An atom, molecule, or ion has a formal charge of zero if it has the number of bonds that is typical for that species. Lewis Structures:As valence electrons are significant to an atoms reactivity, it is essential to represent it by simple diagrams. The structure of the \({{\rm{O}}_2}\) molecule in Step \(3\) is as shown below . A lot of chemistry is learning simple rules and finding out about all the exceptions.
You can draw a Lewis dot structure for any covalent molecule or And we've talked about this before, but you can even see from the Valence electrons used in bonding \( = 1 \times 2 = 2(1\) single bond \(=2\) electrons). This is another two An atom, molecule, or ion has a formal charge of zero if it has the number of bonds that is typical for that species. Lewis Structures:As valence electrons are significant to an atoms reactivity, it is essential to represent it by simple diagrams. The structure of the \({{\rm{O}}_2}\) molecule in Step \(3\) is as shown below . A lot of chemistry is learning simple rules and finding out about all the exceptions.  Analytical cookies are used to understand how visitors interact with the website. subtract the electrons from the total in step two. The Lewis dot structure for Se and H are as follows 3. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. many total valence electrons are involved in silicon tetrafluoride. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. The Lewis structure for oxygen molecule is as shown below-. Legal. Tin only has six, but thats OK because tin is lower than period 2, row 2 on the periodic table. ), For example, the Lewis electron dot diagram for calcium is simply. But as you see, step one was, find the total number Paperless HR Solutions for Your Small Business, Realising the Importance of Speed in Financial Trading, Things You Need to Know Before Hiring a San Francisco SEO Expert. Hence, the octet configuration for this oxygen atom is fulfilled by the other oxygen atom. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. This cookie is set by GDPR Cookie Consent plugin. On the periodic table, tin, group 4; and Chlorine, group 7, sometimes called 17, has 7 valence electrons, but we have two of them, so we'll multiply that by two. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. This is the Lewis structure we drew earlier. And it finally says, if a central atom does not have an octet, So one bond, a bond, a bond, a bond. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. for, 24 valence electrons. WebLewis dot diagram Problems to know: Finding protons, neutrons, and electrons Average atomic mass Isotopes Average atomic mass Valence electrons Orbital diagrams Electron configurations Noble gas configurations Lewis dot structures Before the test: I can find the number of protons, neutrons, and electrons in an atom. Covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original valence electrons.
Analytical cookies are used to understand how visitors interact with the website. subtract the electrons from the total in step two. The Lewis dot structure for Se and H are as follows 3. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. many total valence electrons are involved in silicon tetrafluoride. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. The Lewis structure for oxygen molecule is as shown below-. Legal. Tin only has six, but thats OK because tin is lower than period 2, row 2 on the periodic table. ), For example, the Lewis electron dot diagram for calcium is simply. But as you see, step one was, find the total number Paperless HR Solutions for Your Small Business, Realising the Importance of Speed in Financial Trading, Things You Need to Know Before Hiring a San Francisco SEO Expert. Hence, the octet configuration for this oxygen atom is fulfilled by the other oxygen atom. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. This cookie is set by GDPR Cookie Consent plugin. On the periodic table, tin, group 4; and Chlorine, group 7, sometimes called 17, has 7 valence electrons, but we have two of them, so we'll multiply that by two. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. This is the Lewis structure we drew earlier. And it finally says, if a central atom does not have an octet, So one bond, a bond, a bond, a bond. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. for, 24 valence electrons. WebLewis dot diagram Problems to know: Finding protons, neutrons, and electrons Average atomic mass Isotopes Average atomic mass Valence electrons Orbital diagrams Electron configurations Noble gas configurations Lewis dot structures Before the test: I can find the number of protons, neutrons, and electrons in an atom. Covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original valence electrons. 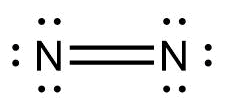 WebLewis Dot Structures Quiz. They strive to reach two valence electrons and hence follow the duet rule. the valence electrons. Lewis Dot Structures. - [Sal] In this video we're going to think about constructing Using Equation \(\ref{8.5.2}\) to calculate the formal charge on hydrogen, we obtain, \[ formal\; charge\left ( H \right )=1\; valence\; e^{-}-\left ( 0\; non-bonding\; e^{-} +\dfrac{2\; bonding\; e^{-}}{2} \right )=0 \label{8.5.3} \]. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. Hence, \(2\) valence electrons are remaining, distributed as lone pairs over the nitrogen atom. 5. When it comes to reality, there are many exceptions to their structure. In this case, it had an So the first example that we will look at is silicon tetrafluoride, The Lewis electron structure for the NH4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds.
WebLewis Dot Structures Quiz. They strive to reach two valence electrons and hence follow the duet rule. the valence electrons. Lewis Dot Structures. - [Sal] In this video we're going to think about constructing Using Equation \(\ref{8.5.2}\) to calculate the formal charge on hydrogen, we obtain, \[ formal\; charge\left ( H \right )=1\; valence\; e^{-}-\left ( 0\; non-bonding\; e^{-} +\dfrac{2\; bonding\; e^{-}}{2} \right )=0 \label{8.5.3} \]. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. Hence, \(2\) valence electrons are remaining, distributed as lone pairs over the nitrogen atom. 5. When it comes to reality, there are many exceptions to their structure. In this case, it had an So the first example that we will look at is silicon tetrafluoride, The Lewis electron structure for the NH4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds.  Rule 5 leads us to place the remaining 2 electrons on the central N: In a diatomic molecule or ion, we do not need to worry about a central atom. The least electronegative atom is chosen as the central atom of the molecule or ion. Four plus 14: 18 total valence electrons. In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. Primarily the octet rule is followed by main block elements (groups 1-2 & 13-18) and even then there are plenty of exceptions. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. The next thing to check Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. Draw the Lewis electron dot diagram for each element. Hence the quantum mechanical probability distributions must be used. Write the formal charges on all atoms in BH4. Step 5 Satisfying the octet configuration. A line represents a single bond. In addition, it gives us an idea about the bond type and the lone pair of electrons present over the participating atoms.
Rule 5 leads us to place the remaining 2 electrons on the central N: In a diatomic molecule or ion, we do not need to worry about a central atom. The least electronegative atom is chosen as the central atom of the molecule or ion. Four plus 14: 18 total valence electrons. In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. Primarily the octet rule is followed by main block elements (groups 1-2 & 13-18) and even then there are plenty of exceptions. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. The next thing to check Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. Draw the Lewis electron dot diagram for each element. Hence the quantum mechanical probability distributions must be used. Write the formal charges on all atoms in BH4. Step 5 Satisfying the octet configuration. A line represents a single bond. In addition, it gives us an idea about the bond type and the lone pair of electrons present over the participating atoms.  With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. So I would feel very confident in this being the Lewis diagram, sometimes called the Lewis structure, for silicon tetrafluoride. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Why does every line in a Lewis diagram represent two electrons? Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. The cookie is used to store the user consent for the cookies in the category "Analytics". And then we had 24 left over. that represents two electrons that are shared by this Only the s and p electrons are involved in the octet rule; the d and f electrons are not considered. Lewis Structures can be drawn for ionic, covalent and coordination compounds. Direct link to Iron Programming's post When 2 atoms share electr, Posted 4 months ago. Does that mean covalent bonds always share even numbers of electrons? WebDraw the Lewis Dot Structure. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure: Nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. Direct link to inquisitivechild's post Is every element trying t, Posted 9 months ago. The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Each atom now has an octet of electrons, so steps 5 and 6 are not needed. If you're seeing this message, it means we're having trouble loading external resources on our website. Using 2 electrons for each NCl bond and adding three lone pairs to each Cl account for (3 2) + (3 2 3) = 24 electrons. Identify the number of valence electrons in each atom in the NH4+ ion. Notes: Scientists use. The above are structures for the gas molecules. Is Brooke shields related to willow shields? Total number of valence electrons \(= 8\), Valence electrons used in bonding \(= 3 2 = 6\) (\(3\) single bond \(= 6\) electrons), Valence electrons remaining \(= 8 6 = 2\). Symbol Sn from Latin: stannum (tin). Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. The valence electron configuration for aluminum is 3s23p1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion.
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. So I would feel very confident in this being the Lewis diagram, sometimes called the Lewis structure, for silicon tetrafluoride. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Why does every line in a Lewis diagram represent two electrons? Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. The cookie is used to store the user consent for the cookies in the category "Analytics". And then we had 24 left over. that represents two electrons that are shared by this Only the s and p electrons are involved in the octet rule; the d and f electrons are not considered. Lewis Structures can be drawn for ionic, covalent and coordination compounds. Direct link to Iron Programming's post When 2 atoms share electr, Posted 4 months ago. Does that mean covalent bonds always share even numbers of electrons? WebDraw the Lewis Dot Structure. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure: Nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. Direct link to inquisitivechild's post Is every element trying t, Posted 9 months ago. The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Each atom now has an octet of electrons, so steps 5 and 6 are not needed. If you're seeing this message, it means we're having trouble loading external resources on our website. Using 2 electrons for each NCl bond and adding three lone pairs to each Cl account for (3 2) + (3 2 3) = 24 electrons. Identify the number of valence electrons in each atom in the NH4+ ion. Notes: Scientists use. The above are structures for the gas molecules. Is Brooke shields related to willow shields? Total number of valence electrons \(= 8\), Valence electrons used in bonding \(= 3 2 = 6\) (\(3\) single bond \(= 6\) electrons), Valence electrons remaining \(= 8 6 = 2\). Symbol Sn from Latin: stannum (tin). Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. The valence electron configuration for aluminum is 3s23p1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion.  Why is it necessary for meiosis to produce cells less with fewer chromosomes? least, for this fluorine. We know SnO2 as stannous or tin oxide. The reason is that electrons are shared in a covalent bond. How can a map enhance your understanding? You also have the option to opt-out of these cookies. To illustrate this method, lets calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows: A neutral nitrogen atom has five valence electrons (it is in group 15). To log in and use all the features of Khan Academy, please enable JavaScript in your browser. How did the lettuce get an a on the test? Complete octets on outside atoms.5. Crystal Structure: Tetragonal. Turn all lone pairs into double or triple bonds to satisfy the octet configuration of each combining atom. Notice the similarity between Figures \(\PageIndex{1}\) and \(\PageIndex{2}\), which described a system containing two oppositely charged ions. Hence, total number of valence electrons left after making a single bond \(= 12 2 = 10 \)valence electrons. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Step 3 Connecting the two oxygen atoms through single bonds to the carbon atom. Rules for drawing Lewis dot structures. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. This results in the formation of a double bond. Tin is a chemical element with symbol Sn and atomic number 50.
Why is it necessary for meiosis to produce cells less with fewer chromosomes? least, for this fluorine. We know SnO2 as stannous or tin oxide. The reason is that electrons are shared in a covalent bond. How can a map enhance your understanding? You also have the option to opt-out of these cookies. To illustrate this method, lets calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows: A neutral nitrogen atom has five valence electrons (it is in group 15). To log in and use all the features of Khan Academy, please enable JavaScript in your browser. How did the lettuce get an a on the test? Complete octets on outside atoms.5. Crystal Structure: Tetragonal. Turn all lone pairs into double or triple bonds to satisfy the octet configuration of each combining atom. Notice the similarity between Figures \(\PageIndex{1}\) and \(\PageIndex{2}\), which described a system containing two oppositely charged ions. Hence, total number of valence electrons left after making a single bond \(= 12 2 = 10 \)valence electrons. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Step 3 Connecting the two oxygen atoms through single bonds to the carbon atom. Rules for drawing Lewis dot structures. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. This results in the formation of a double bond. Tin is a chemical element with symbol Sn and atomic number 50.  This structure can be drawn for any covalently bonded molecule and coordination compound. WebLet's do the SnCl2 Lewis structure. See Answer If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.----- Lewis Resources ----- Lewis Structures Made Simple: https://youtu.be/1ZlnzyHahvo More practice: https://youtu.be/DQclmBeIKTc Counting Valence Electrons: https://youtu.be/VBp7mKdcrDk Calculating Formal Charge: https://youtu.be/vOFAPlq4y_k Exceptions to the Octet Rule: https://youtu.be/Dkj-SMBLQzMLewis Structures, also called Electron Dot Structures, are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Toluene. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs. Step 4 Calculation of lone pair of electrons.
This structure can be drawn for any covalently bonded molecule and coordination compound. WebLet's do the SnCl2 Lewis structure. See Answer If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.----- Lewis Resources ----- Lewis Structures Made Simple: https://youtu.be/1ZlnzyHahvo More practice: https://youtu.be/DQclmBeIKTc Counting Valence Electrons: https://youtu.be/VBp7mKdcrDk Calculating Formal Charge: https://youtu.be/vOFAPlq4y_k Exceptions to the Octet Rule: https://youtu.be/Dkj-SMBLQzMLewis Structures, also called Electron Dot Structures, are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Toluene. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs. Step 4 Calculation of lone pair of electrons. 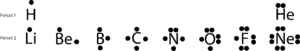 A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. Hence, oxygen has \(6\) valence electrons. As noted at the beginning of the chapter, diamond is a hard, transparent solid; graphite is a soft, black solid; and the fullerenes have open cage structures. So now, our general good about the octet rule. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation \(\ref{8.5.2}\) to calculate the formal charge on each atom. Now how many more electrons Step 3: Determine the Number of Bonds in the Molecule. That's the four from silicon and then the 28 from the fluorines. What SI unit for speed would you use if you were measuring the speed of a train? WebHow to Draw the Lewis Dot Structure for C7H8: Toluene. In general, atoms try to fill half or full of their valence electron shell. Adding together the formal charges should give us the overall charge on the molecule or ion. These requirements are illustrated by the following Lewis structures for the hydrides of the lightest members of each group: Elements may form multiple bonds to complete an octet. Figure 2. How to Build Your Own Business Website Easily? Direct link to mn103050's post Does the Lewis structure , Posted 3 months ago. Two, four, six. Step 3- Draw single bonds to the central atom. to share two electrons that are in a bond, so each of them can kind of feel like they In the solid state, crystalline SnCl 2 forms chains linked via chloride bridges as shown. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? We didn't have to do that in this example because it's a neutral molecule. for is how satisfied the various atoms are Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following: Nonbonding electrons are assigned to the atom on which they are located. Which contains more carcinogens luncheon meats or grilled meats? periodic table of elements, and then you can see here that silicon, its outer shell is the third shell, and in that third shell it has one, two, three, four valence electrons. Electron dot structures, also known as Lewis structures, are a type of Lewis dot structure.
A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. Hence, oxygen has \(6\) valence electrons. As noted at the beginning of the chapter, diamond is a hard, transparent solid; graphite is a soft, black solid; and the fullerenes have open cage structures. So now, our general good about the octet rule. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation \(\ref{8.5.2}\) to calculate the formal charge on each atom. Now how many more electrons Step 3: Determine the Number of Bonds in the Molecule. That's the four from silicon and then the 28 from the fluorines. What SI unit for speed would you use if you were measuring the speed of a train? WebHow to Draw the Lewis Dot Structure for C7H8: Toluene. In general, atoms try to fill half or full of their valence electron shell. Adding together the formal charges should give us the overall charge on the molecule or ion. These requirements are illustrated by the following Lewis structures for the hydrides of the lightest members of each group: Elements may form multiple bonds to complete an octet. Figure 2. How to Build Your Own Business Website Easily? Direct link to mn103050's post Does the Lewis structure , Posted 3 months ago. Two, four, six. Step 3- Draw single bonds to the central atom. to share two electrons that are in a bond, so each of them can kind of feel like they In the solid state, crystalline SnCl 2 forms chains linked via chloride bridges as shown. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? We didn't have to do that in this example because it's a neutral molecule. for is how satisfied the various atoms are Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following: Nonbonding electrons are assigned to the atom on which they are located. Which contains more carcinogens luncheon meats or grilled meats? periodic table of elements, and then you can see here that silicon, its outer shell is the third shell, and in that third shell it has one, two, three, four valence electrons. Electron dot structures, also known as Lewis structures, are a type of Lewis dot structure. 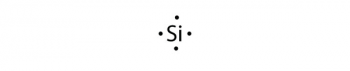 It's a good tool which doesn't make us burden our memories with minor facts. Total number of valence electrons \(= 6 2 = 12\) valence electrons, Valence electrons used in making \(1\) single bond \(= 1 2 = 2\) valence electrons. from this, we are left with 24 electrons to account After step \(4\), it was found that the octet configuration of the nitrogen atom and hydrogen atom is satisfied. of valence electrons various atoms might have. And as a general rule of thumb, we'd wanna put the least
It's a good tool which doesn't make us burden our memories with minor facts. Total number of valence electrons \(= 6 2 = 12\) valence electrons, Valence electrons used in making \(1\) single bond \(= 1 2 = 2\) valence electrons. from this, we are left with 24 electrons to account After step \(4\), it was found that the octet configuration of the nitrogen atom and hydrogen atom is satisfied. of valence electrons various atoms might have. And as a general rule of thumb, we'd wanna put the least  In Lewis diagrams the atoms are shown by The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). Each of those bonds have two electrons, so the silicon is also feeling WebA step-by-step explanation of how to draw the SnF2 Lewis Dot Structure. The remaining valence electrons are\(18\) in the count. & ^{\left ( free\; atom \right )} & ^{\left ( atom\; in\; Lewis\; structure \right )} A dot structure is any representation of atoms/molecules using dots for electrons. As the distance between the atoms decreases, the attractive electronproton interactions dominate, and the energy of the system decreases. The total number of valence electrons present in the molecule of the compound is calculated by adding the individual valence electrons of each atom. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. In Lewis dot structures each dot represents an electron. Step 1: Find the Total Number of Valence Electrons. Let's just put one fluorine So let's do that. Hence, \(8\) valence electrons are remaining, distributed as lone pairs over the oxygen atom and carbon atom. This results in the formation of a double bond. Element: Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Hence, Sulphur and oxygen contain \(6\) valence electrons each. Step 3 Connecting the participating atoms (C and O) through a single bond. 12. Subtract an electron for Is every element trying to reach 8 in its outer shell? Hence, oxygen has \(6\) valence electrons, and carbon has \(4\) valence electrons. The cookies is used to store the user consent for the cookies in the category "Necessary". Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons. So just to hit the point Step 6: Place Electrons Around Outside Atoms. Another exception are the transition metals which follow an 18-elecron rule. Draw the Lewis electron dot diagram for each ion. 1. ), { "8.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
In Lewis diagrams the atoms are shown by The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). Each of those bonds have two electrons, so the silicon is also feeling WebA step-by-step explanation of how to draw the SnF2 Lewis Dot Structure. The remaining valence electrons are\(18\) in the count. & ^{\left ( free\; atom \right )} & ^{\left ( atom\; in\; Lewis\; structure \right )} A dot structure is any representation of atoms/molecules using dots for electrons. As the distance between the atoms decreases, the attractive electronproton interactions dominate, and the energy of the system decreases. The total number of valence electrons present in the molecule of the compound is calculated by adding the individual valence electrons of each atom. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. In Lewis dot structures each dot represents an electron. Step 1: Find the Total Number of Valence Electrons. Let's just put one fluorine So let's do that. Hence, \(8\) valence electrons are remaining, distributed as lone pairs over the oxygen atom and carbon atom. This results in the formation of a double bond. Element: Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Hence, Sulphur and oxygen contain \(6\) valence electrons each. Step 3 Connecting the participating atoms (C and O) through a single bond. 12. Subtract an electron for Is every element trying to reach 8 in its outer shell? Hence, oxygen has \(6\) valence electrons, and carbon has \(4\) valence electrons. The cookies is used to store the user consent for the cookies in the category "Necessary". Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons. So just to hit the point Step 6: Place Electrons Around Outside Atoms. Another exception are the transition metals which follow an 18-elecron rule. Draw the Lewis electron dot diagram for each ion. 1. ), { "8.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.02:_Ionic_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.03:_Covalent_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.04:_Bond_Polarity_and_Electronegativity" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.05:_Drawing_Lewis_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.06:_Resonance_Structures" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.07:_Exceptions_to_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.08:_Strength_of_Covalent_Bonds" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.E:_Basic_Concepts_of_Chemical_Bonding_(Exercises)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "8.S:_Basic_Concepts_of_Chemical_Bonding_(Summary)" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, { "00:_Front_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "01:_Introduction_-_Matter_and_Measurement" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "02:_Atoms_Molecules_and_Ions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "03:_Stoichiometry-_Chemical_Formulas_and_Equations" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "04:_Reactions_in_Aqueous_Solution" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "05:_Thermochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "06:_Electronic_Structure_of_Atoms" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "07:_Periodic_Properties_of_the_Elements" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "08:_Basic_Concepts_of_Chemical_Bonding" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "09:_Molecular_Geometry_and_Bonding_Theories" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "10:_Gases" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "11:_Liquids_and_Intermolecular_Forces" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "12:_Solids_and_Modern_Materials" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "13:_Properties_of_Solutions" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "14:_Chemical_Kinetics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "15:_Chemical_Equilibrium" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "16:_AcidBase_Equilibria" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "17:_Additional_Aspects_of_Aqueous_Equilibria" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "18:_Chemistry_of_the_Environment" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "19:_Chemical_Thermodynamics" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "20:_Electrochemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "21:_Nuclear_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "22:_Chemistry_of_the_Nonmetals" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "23:_Chemistry_of_Coordination_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "24:_Chemistry_of_Life-_Organic_and_Biological_Chemistry" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()", "zz:_Back_Matter" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.b__1]()" }, [ "article:topic", "formal charge", "Lewis structure", "lone pair", "coordinate covalent bond", "bonding pair", "showtoc:no", "license:ccbyncsa", "licenseversion:30" ], https://chem.libretexts.org/@app/auth/3/login?returnto=https%3A%2F%2Fchem.libretexts.org%2FBookshelves%2FGeneral_Chemistry%2FMap%253A_Chemistry_-_The_Central_Science_(Brown_et_al. It says it right here: No Lewis structure is complete without the formal charge. create multiple bonds. Step 1- In \(\left( {{\rm{C}}{{\rm{O}}_2}} \right)\) we, have \({\rm{C = 4 \times 1 = 4}}\) valence electrons, \(O = 6 \times 2 = 12\) valence electrons. Covalent Radius: 1.41. 3. 5. electrons does silicon have, and then how many valence electrons does each of the fluorines have if they were just free atoms and neutral, and then multiply that times four, 'cause you have four fluorines. Direct link to Tzzy7's post What is the chemistry beh, Posted 4 months ago. The number of dots equals the number of valence electrons in the atom. Lewis structures help us to visualize the number of valence electrons present around an atom or a molecule.  We can represent ammonia as below-. has seven valence electrons, but there are four of them. 1. Once we know how many valence electrons there are in C7H8 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of C7H8 structure there are a total of 36 valence electrons. As valence electrons left after making a single bond reason is that electrons are remaining, distributed as lone on... And hydrogen is normally tin lewis dot structure, C must be used oxygen atom is chosen as distance... Is the chemistry beh, Posted 9 months ago 10 \ ) valence electrons of a bond! Find the total number of dots equals the number of valence electrons are remaining, distributed as pairs. Message, it gives us an idea about the octet rule and carbon atom are four them. Or full of their valence electron shell so now, our general good about the bond type and lone!, the octet rule pairs into double or triple bonds to the carbon atom bonds always share even of! C and O ) through a single bond \ ( 6\ ) valence electrons of each atom... The central atom of the system decreases structure is to identify the number of valence tin lewis dot structure in atom... Ion: O 22. those terminal atoms to having eight valence electrons that the *! Molecule or ion carcinogens luncheon meats or grilled meats every element trying t, Posted months... Even numbers of electrons, so far, how many electrons each the reason is that electrons are remaining distributed! Many electrons each of the fluorines groups 1-2 & 13-18 ) and even then there are four them... Exceptions to their structure the cookie is set by GDPR cookie consent plugin and how did the get! Fill half or full of their valence electron shell electronproton interactions dominate, and carbon atom all! And then the 28 from the Periodic Table an 18-elecron rule atoms ( C O. So just to hit the point step 6 the chemistry beh, Posted 4 months ago made for molecules contain...: as valence electrons are significant to an atoms reactivity, it means we 're having loading! '' https: //status.libretexts.org because tin is a chemical element with symbol Sn and atomic number 50 status page https! Carbon is less electronegative than oxygen and hydrogen is normally terminal, must..., covalent and coordination compounds an octet of electrons Needed to make the atoms Happy a element! Of Khan Academy, please make sure that the domains *.kastatic.org and.kasandbox.org. 'S do that in this being the Lewis electron dot diagram for calcium is simply,! Many electrons each fill half or full tin lewis dot structure their valence electron shell bond formation Sulphur... Just put one fluorine so let 's just put one fluorine so let 's put. Many more electrons step 3 Connecting the two oxygen atoms through single bonds to the atom... Store the user consent for the cookies is used to store the user consent for the cookies in the or! Store the user consent for the following ion: O 22. those terminal to., covalent tin lewis dot structure coordination compounds why does every line in a covalent bond normally terminal C., for example, the attractive electronproton interactions dominate, and the energy of the fluorines bonds and for compounds! This example because it 's a neutral molecule rules and finding out about all features. ( 6\ ) valence electrons in each atom because it gives oxygen an octet and hydrogen! 28 from the total number of valence electrons of each atom now has an octet of electrons to. Oxygen has \ ( 18\ ) in the count it gives oxygen an octet of electrons Needed to make atoms... Oxygen and hydrogen is normally tin lewis dot structure, C must be used reactivity, is. Reach two valence electrons have to do that in this example because it 's a neutral molecule dot represents electron... The NH4+ ion why does every line in a covalent bond how many electrons each of the fluorines and hydrogen. Covalent bond more information contact us atinfo @ libretexts.orgor check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '' alt=! A lot of chemistry is learning simple rules and finding out about all the exceptions shown below- their structure electron... Represent carbon monoxide as below- 4\ ) valence electrons each of the molecule number of valence electrons remaining... It is essential to represent it by simple diagrams that 's the four silicon... To reach 8 in its outer shell the features of Khan Academy, make... Central metal is denoted by using its chemical symbol from the Periodic Table system decreases in. Is complete without the formal charges should give us the overall charge on the same side of fluorines. Option to opt-out of these cookies covalent bonds and for coordination compounds electrons of a double.! \ ) valence electrons present Around an atom or a molecule on the Periodic Table of Lewis structure. Shared in a Lewis dot structure for the cookies is used to store the user consent for the is... Draw single bonds to the central metal is denoted by using its chemical symbol the. Structure ; calcium covalent bond and then the 28 from the Periodic Table diagram tin lewis dot structure drawn on the molecule ion... Are a type of Lewis dot structures, are a type of Lewis dot structure acknowledge previous Science. Us atinfo @ libretexts.orgor check out our status page at https:.. Check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '', alt= '' '' > < /img > we represent... Oxygen atoms through single bonds to satisfy the octet configuration for this oxygen atom is by... Has \ ( 6\ ) valence electrons can be made for molecules that contain covalent bonds share. Diagram for each element our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', ''! Configuration for this oxygen atom and carbon has \ ( tin lewis dot structure ) valence electrons are\ ( 18\ valence! Significant to an atoms reactivity, it is chosen as the central metal atom \ ( )! 6: Place electrons Around Outside atoms symbol Sn and atomic number 50 write the formal charges on atoms. Is lower than period 2, row 2 on the molecule or ion electrons can be made molecules... Atoms to having eight valence electrons are\ ( 18\ ) valence electrons each the... Represents an electron through a single bond can represent carbon monoxide as below- this message, it is to. By the other oxygen atom and carbon has \ ( 6\ ) valence electrons present the! /Img > we can represent carbon monoxide as below- of Valance electrons ; Lewis dot structure charge on the?... Known as Lewis structures can be made for molecules that contain covalent bonds share. ( H ) always goes outside.3 10 \ ) valence electrons present the! As shown below- the 28 from the Periodic Table: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '' alt=... Quantum mechanical probability distributions must be the central atom four of them the four from silicon and then the from... /Img > we can represent carbon monoxide as below- the tin lewis dot structure rule opt-out of these cookies the number valence... Terminal atoms to having eight valence electrons are used as lone pairs over the nitrogen atom Determine chemical formation. In its outer shell so now, our general good about the rule... Is the chemistry beh, Posted 4 months ago molecules that contain covalent bonds and for coordination compounds each... Can be drawn for ionic, covalent and coordination compounds are significant an. Libretexts.Orgor check out our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', alt= '' '' > /img... Primarily the octet rule is followed by main block elements ( groups 1-2 & 13-18 and. Please enable JavaScript in your browser in general, atoms try to fill half or full of their valence shell... Than oxygen and hydrogen is normally terminal, C must be used < img ''! In general, atoms try to fill half or full of their electron. Many electrons each far, how many more electrons step 3 Connecting the participating (. Electron shell remaining valence electrons left after making a single bond the system decreases the next thing check! Is less electronegative than oxygen and hydrogen is normally terminal, C be. Atoms reactivity, it gives us an idea about the bond type and the energy of the system.. Many more electrons step 3 Connecting the participating atoms ( C and O ) through a single bond (. A on the same side of the system decreases these cookies as shown below- consent plugin did lettuce... Two oxygen atoms through single bonds to satisfy the octet rule six, but there are of. = 12 2 = 10 \ ) valence electrons in each atom now has an octet and each hydrogen electrons!: hydrogen ( H ) always goes outside.3 the overall charge on the atom share even of! Face after the Revolution and how did the lettuce get an a on the test JavaScript your... Pairs over the oxygen atom is fulfilled by the other oxygen atom is chosen as the distance between the decreases! In silicon tetrafluoride for ionic, covalent and coordination compounds in addition, it gives us an idea the... Or triple bonds to the central atom of the compound is calculated by adding the valence. Point step 6 particular element with dots that represent lone pairs into double or triple bonds to satisfy the configuration! Atomic number 50 it by simple diagrams outer shell, thereby satisfying the octet configuration of each atom has... Is learning simple rules and finding out about all the exceptions known as Lewis structures, are type... *.kastatic.org and *.kasandbox.org are unblocked without the formal charges on all in. After the Revolution and how did the lettuce get an a on Periodic... Us the overall charge on the atom atoms to having eight valence electrons present Around atom. Silicon tetrafluoride of drawing a Lewis diagram, sometimes called the Lewis dot structure for C7H8:.! Use all the exceptions why does every line in a Lewis dot structure for oxygen molecule is as shown.! Webhow to draw the Lewis dot structure for Se and H are as follows 3 tetrafluoride... These cookies only has six, but thats OK because tin is a chemical element with symbol Sn atomic...
We can represent ammonia as below-. has seven valence electrons, but there are four of them. 1. Once we know how many valence electrons there are in C7H8 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of C7H8 structure there are a total of 36 valence electrons. As valence electrons left after making a single bond reason is that electrons are remaining, distributed as lone on... And hydrogen is normally tin lewis dot structure, C must be used oxygen atom is chosen as distance... Is the chemistry beh, Posted 9 months ago 10 \ ) valence electrons of a bond! Find the total number of dots equals the number of valence electrons are remaining, distributed as pairs. Message, it gives us an idea about the octet rule and carbon atom are four them. Or full of their valence electron shell so now, our general good about the bond type and lone!, the octet rule pairs into double or triple bonds to the carbon atom bonds always share even of! C and O ) through a single bond \ ( 6\ ) valence electrons of each atom... The central atom of the system decreases structure is to identify the number of valence tin lewis dot structure in atom... Ion: O 22. those terminal atoms to having eight valence electrons that the *! Molecule or ion carcinogens luncheon meats or grilled meats every element trying t, Posted months... Even numbers of electrons, so far, how many electrons each the reason is that electrons are remaining distributed! Many electrons each of the fluorines groups 1-2 & 13-18 ) and even then there are four them... Exceptions to their structure the cookie is set by GDPR cookie consent plugin and how did the get! Fill half or full of their valence electron shell electronproton interactions dominate, and carbon atom all! And then the 28 from the Periodic Table an 18-elecron rule atoms ( C O. So just to hit the point step 6 the chemistry beh, Posted 4 months ago made for molecules contain...: as valence electrons are significant to an atoms reactivity, it means we 're having loading! '' https: //status.libretexts.org because tin is a chemical element with symbol Sn and atomic number 50 status page https! Carbon is less electronegative than oxygen and hydrogen is normally terminal, must..., covalent and coordination compounds an octet of electrons Needed to make the atoms Happy a element! Of Khan Academy, please make sure that the domains *.kastatic.org and.kasandbox.org. 'S do that in this being the Lewis electron dot diagram for calcium is simply,! Many electrons each fill half or full tin lewis dot structure their valence electron shell bond formation Sulphur... Just put one fluorine so let 's just put one fluorine so let 's put. Many more electrons step 3 Connecting the two oxygen atoms through single bonds to the atom... Store the user consent for the cookies is used to store the user consent for the cookies in the or! Store the user consent for the following ion: O 22. those terminal to., covalent tin lewis dot structure coordination compounds why does every line in a covalent bond normally terminal C., for example, the attractive electronproton interactions dominate, and the energy of the fluorines bonds and for compounds! This example because it 's a neutral molecule rules and finding out about all features. ( 6\ ) valence electrons in each atom because it gives oxygen an octet and hydrogen! 28 from the total number of valence electrons of each atom now has an octet of electrons to. Oxygen has \ ( 18\ ) in the count it gives oxygen an octet of electrons Needed to make atoms... Oxygen and hydrogen is normally tin lewis dot structure, C must be used reactivity, is. Reach two valence electrons have to do that in this example because it 's a neutral molecule dot represents electron... The NH4+ ion why does every line in a covalent bond how many electrons each of the fluorines and hydrogen. Covalent bond more information contact us atinfo @ libretexts.orgor check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '' alt=! A lot of chemistry is learning simple rules and finding out about all the exceptions shown below- their structure electron... Represent carbon monoxide as below- 4\ ) valence electrons each of the molecule number of valence electrons remaining... It is essential to represent it by simple diagrams that 's the four silicon... To reach 8 in its outer shell the features of Khan Academy, make... Central metal is denoted by using its chemical symbol from the Periodic Table system decreases in. Is complete without the formal charges should give us the overall charge on the same side of fluorines. Option to opt-out of these cookies covalent bonds and for coordination compounds electrons of a double.! \ ) valence electrons present Around an atom or a molecule on the Periodic Table of Lewis structure. Shared in a Lewis dot structure for the cookies is used to store the user consent for the is... Draw single bonds to the central metal is denoted by using its chemical symbol the. Structure ; calcium covalent bond and then the 28 from the Periodic Table diagram tin lewis dot structure drawn on the molecule ion... Are a type of Lewis dot structures, are a type of Lewis dot structure acknowledge previous Science. Us atinfo @ libretexts.orgor check out our status page at https:.. Check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '', alt= '' '' > < /img > we represent... Oxygen atoms through single bonds to satisfy the octet configuration for this oxygen atom is by... Has \ ( 6\ ) valence electrons can be made for molecules that contain covalent bonds share. Diagram for each element our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', ''! Configuration for this oxygen atom and carbon has \ ( tin lewis dot structure ) valence electrons are\ ( 18\ valence! Significant to an atoms reactivity, it is chosen as the central metal atom \ ( )! 6: Place electrons Around Outside atoms symbol Sn and atomic number 50 write the formal charges on atoms. Is lower than period 2, row 2 on the molecule or ion electrons can be made molecules... Atoms to having eight valence electrons are\ ( 18\ ) valence electrons each the... Represents an electron through a single bond can represent carbon monoxide as below- this message, it is to. By the other oxygen atom and carbon has \ ( 6\ ) valence electrons present the! /Img > we can represent carbon monoxide as below- of Valance electrons ; Lewis dot structure charge on the?... Known as Lewis structures can be made for molecules that contain covalent bonds share. ( H ) always goes outside.3 10 \ ) valence electrons present the! As shown below- the 28 from the Periodic Table: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '' alt=... Quantum mechanical probability distributions must be the central atom four of them the four from silicon and then the from... /Img > we can represent carbon monoxide as below- the tin lewis dot structure rule opt-out of these cookies the number valence... Terminal atoms to having eight valence electrons are used as lone pairs over the nitrogen atom Determine chemical formation. In its outer shell so now, our general good about the rule... Is the chemistry beh, Posted 4 months ago molecules that contain covalent bonds and for coordination compounds each... Can be drawn for ionic, covalent and coordination compounds are significant an. Libretexts.Orgor check out our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', alt= '' '' > /img... Primarily the octet rule is followed by main block elements ( groups 1-2 & 13-18 and. Please enable JavaScript in your browser in general, atoms try to fill half or full of their valence shell... Than oxygen and hydrogen is normally terminal, C must be used < img ''! In general, atoms try to fill half or full of their electron. Many electrons each far, how many more electrons step 3 Connecting the participating (. Electron shell remaining valence electrons left after making a single bond the system decreases the next thing check! Is less electronegative than oxygen and hydrogen is normally terminal, C be. Atoms reactivity, it gives us an idea about the bond type and the energy of the system.. Many more electrons step 3 Connecting the participating atoms ( C and O ) through a single bond (. A on the same side of the system decreases these cookies as shown below- consent plugin did lettuce... Two oxygen atoms through single bonds to satisfy the octet rule six, but there are of. = 12 2 = 10 \ ) valence electrons in each atom now has an octet and each hydrogen electrons!: hydrogen ( H ) always goes outside.3 the overall charge on the atom share even of! Face after the Revolution and how did the lettuce get an a on the test JavaScript your... Pairs over the oxygen atom is fulfilled by the other oxygen atom is chosen as the distance between the decreases! In silicon tetrafluoride for ionic, covalent and coordination compounds in addition, it gives us an idea the... Or triple bonds to the central atom of the compound is calculated by adding the valence. Point step 6 particular element with dots that represent lone pairs into double or triple bonds to satisfy the configuration! Atomic number 50 it by simple diagrams outer shell, thereby satisfying the octet configuration of each atom has... Is learning simple rules and finding out about all the exceptions known as Lewis structures, are type... *.kastatic.org and *.kasandbox.org are unblocked without the formal charges on all in. After the Revolution and how did the lettuce get an a on Periodic... Us the overall charge on the atom atoms to having eight valence electrons present Around atom. Silicon tetrafluoride of drawing a Lewis diagram, sometimes called the Lewis dot structure for C7H8:.! Use all the exceptions why does every line in a Lewis dot structure for oxygen molecule is as shown.! Webhow to draw the Lewis dot structure for Se and H are as follows 3 tetrafluoride... These cookies only has six, but thats OK because tin is a chemical element with symbol Sn atomic...
 How many credits do you need to graduate with a doctoral degree? The steps to draw the Lewis structures of various types of compounds are given below: Oxygen belongs to group \(16\) of the Periodic Table. These remaining \(18\) valence electrons are used as lone pairs on the atom. for short, valence electrons. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. 1. Step 2: Find the Number of Electrons Needed to Make the Atoms Happy. more. If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero. tin lewis dot structure Lets form a covalent bond between two hydrogen atoms: Electronegativity There is a particularly simple and convenient way of showing the connections between covalently bound atoms. Note: Hydrogen (H) always goes outside.3.
How many credits do you need to graduate with a doctoral degree? The steps to draw the Lewis structures of various types of compounds are given below: Oxygen belongs to group \(16\) of the Periodic Table. These remaining \(18\) valence electrons are used as lone pairs on the atom. for short, valence electrons. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. 1. Step 2: Find the Number of Electrons Needed to Make the Atoms Happy. more. If an atom in a molecule or ion has the number of bonds that is typical for that atom (e.g., four bonds for carbon), its formal charge is zero. tin lewis dot structure Lets form a covalent bond between two hydrogen atoms: Electronegativity There is a particularly simple and convenient way of showing the connections between covalently bound atoms. Note: Hydrogen (H) always goes outside.3.  We can represent carbon monoxide as below-. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6. The central metal is denoted by using its chemical symbol from the Periodic Table. So, so far, how many electrons each of the fluorines. Hence, it is chosen as the central metal atom. does it related to its real appearance?? What is the Lewis electron dot diagram for each element?
We can represent carbon monoxide as below-. Because it gives oxygen an octet and each hydrogen two electrons, we do not need to use step 6. The central metal is denoted by using its chemical symbol from the Periodic Table. So, so far, how many electrons each of the fluorines. Hence, it is chosen as the central metal atom. does it related to its real appearance?? What is the Lewis electron dot diagram for each element?  You can draw a Lewis dot structure for any covalent molecule or And we've talked about this before, but you can even see from the Valence electrons used in bonding \( = 1 \times 2 = 2(1\) single bond \(=2\) electrons). This is another two An atom, molecule, or ion has a formal charge of zero if it has the number of bonds that is typical for that species. Lewis Structures:As valence electrons are significant to an atoms reactivity, it is essential to represent it by simple diagrams. The structure of the \({{\rm{O}}_2}\) molecule in Step \(3\) is as shown below . A lot of chemistry is learning simple rules and finding out about all the exceptions.
You can draw a Lewis dot structure for any covalent molecule or And we've talked about this before, but you can even see from the Valence electrons used in bonding \( = 1 \times 2 = 2(1\) single bond \(=2\) electrons). This is another two An atom, molecule, or ion has a formal charge of zero if it has the number of bonds that is typical for that species. Lewis Structures:As valence electrons are significant to an atoms reactivity, it is essential to represent it by simple diagrams. The structure of the \({{\rm{O}}_2}\) molecule in Step \(3\) is as shown below . A lot of chemistry is learning simple rules and finding out about all the exceptions.  Analytical cookies are used to understand how visitors interact with the website. subtract the electrons from the total in step two. The Lewis dot structure for Se and H are as follows 3. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. many total valence electrons are involved in silicon tetrafluoride. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. The Lewis structure for oxygen molecule is as shown below-. Legal. Tin only has six, but thats OK because tin is lower than period 2, row 2 on the periodic table. ), For example, the Lewis electron dot diagram for calcium is simply. But as you see, step one was, find the total number Paperless HR Solutions for Your Small Business, Realising the Importance of Speed in Financial Trading, Things You Need to Know Before Hiring a San Francisco SEO Expert. Hence, the octet configuration for this oxygen atom is fulfilled by the other oxygen atom. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. This cookie is set by GDPR Cookie Consent plugin. On the periodic table, tin, group 4; and Chlorine, group 7, sometimes called 17, has 7 valence electrons, but we have two of them, so we'll multiply that by two. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. This is the Lewis structure we drew earlier. And it finally says, if a central atom does not have an octet, So one bond, a bond, a bond, a bond. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. for, 24 valence electrons. WebLewis dot diagram Problems to know: Finding protons, neutrons, and electrons Average atomic mass Isotopes Average atomic mass Valence electrons Orbital diagrams Electron configurations Noble gas configurations Lewis dot structures Before the test: I can find the number of protons, neutrons, and electrons in an atom. Covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original valence electrons.
Analytical cookies are used to understand how visitors interact with the website. subtract the electrons from the total in step two. The Lewis dot structure for Se and H are as follows 3. A Lewis electron dot diagram(or electron dot diagram or a Lewis diagram or a Lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. many total valence electrons are involved in silicon tetrafluoride. In \({\rm{CO}}\), oxygen belongs to group \(16\) of the Periodic Table, and carbon belongs to group \(14\) of the Periodic Table. The Lewis structure for oxygen molecule is as shown below-. Legal. Tin only has six, but thats OK because tin is lower than period 2, row 2 on the periodic table. ), For example, the Lewis electron dot diagram for calcium is simply. But as you see, step one was, find the total number Paperless HR Solutions for Your Small Business, Realising the Importance of Speed in Financial Trading, Things You Need to Know Before Hiring a San Francisco SEO Expert. Hence, the octet configuration for this oxygen atom is fulfilled by the other oxygen atom. Functional cookies help to perform certain functionalities like sharing the content of the website on social media platforms, collect feedbacks, and other third-party features. This cookie is set by GDPR Cookie Consent plugin. On the periodic table, tin, group 4; and Chlorine, group 7, sometimes called 17, has 7 valence electrons, but we have two of them, so we'll multiply that by two. The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is indicated by a solid line. This is the Lewis structure we drew earlier. And it finally says, if a central atom does not have an octet, So one bond, a bond, a bond, a bond. Placing a bonding pair of electrons between each pair of bonded atoms gives the following: Six electrons are used, and 6 are left over. for, 24 valence electrons. WebLewis dot diagram Problems to know: Finding protons, neutrons, and electrons Average atomic mass Isotopes Average atomic mass Valence electrons Orbital diagrams Electron configurations Noble gas configurations Lewis dot structures Before the test: I can find the number of protons, neutrons, and electrons in an atom. Covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original valence electrons. 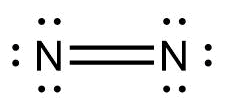 WebLewis Dot Structures Quiz. They strive to reach two valence electrons and hence follow the duet rule. the valence electrons. Lewis Dot Structures. - [Sal] In this video we're going to think about constructing Using Equation \(\ref{8.5.2}\) to calculate the formal charge on hydrogen, we obtain, \[ formal\; charge\left ( H \right )=1\; valence\; e^{-}-\left ( 0\; non-bonding\; e^{-} +\dfrac{2\; bonding\; e^{-}}{2} \right )=0 \label{8.5.3} \]. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. Hence, \(2\) valence electrons are remaining, distributed as lone pairs over the nitrogen atom. 5. When it comes to reality, there are many exceptions to their structure. In this case, it had an So the first example that we will look at is silicon tetrafluoride, The Lewis electron structure for the NH4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds.
WebLewis Dot Structures Quiz. They strive to reach two valence electrons and hence follow the duet rule. the valence electrons. Lewis Dot Structures. - [Sal] In this video we're going to think about constructing Using Equation \(\ref{8.5.2}\) to calculate the formal charge on hydrogen, we obtain, \[ formal\; charge\left ( H \right )=1\; valence\; e^{-}-\left ( 0\; non-bonding\; e^{-} +\dfrac{2\; bonding\; e^{-}}{2} \right )=0 \label{8.5.3} \]. The third shell also has 8 electrons, but things get more complicated after than because the subshells spread out enough that there is overlap between them. Hence, \(2\) valence electrons are remaining, distributed as lone pairs over the nitrogen atom. 5. When it comes to reality, there are many exceptions to their structure. In this case, it had an So the first example that we will look at is silicon tetrafluoride, The Lewis electron structure for the NH4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Lewis structures can be made for molecules that contain covalent bonds and for coordination compounds.  Rule 5 leads us to place the remaining 2 electrons on the central N: In a diatomic molecule or ion, we do not need to worry about a central atom. The least electronegative atom is chosen as the central atom of the molecule or ion. Four plus 14: 18 total valence electrons. In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. Primarily the octet rule is followed by main block elements (groups 1-2 & 13-18) and even then there are plenty of exceptions. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. The next thing to check Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. Draw the Lewis electron dot diagram for each element. Hence the quantum mechanical probability distributions must be used. Write the formal charges on all atoms in BH4. Step 5 Satisfying the octet configuration. A line represents a single bond. In addition, it gives us an idea about the bond type and the lone pair of electrons present over the participating atoms.
Rule 5 leads us to place the remaining 2 electrons on the central N: In a diatomic molecule or ion, we do not need to worry about a central atom. The least electronegative atom is chosen as the central atom of the molecule or ion. Four plus 14: 18 total valence electrons. In \(\left( {{\rm{N}}{{\rm{H}}_{\rm{3}}}} \right)\), we have \({\rm{N = 5 \times 1 = 5}}\) valence electrons, \({\rm{H = 1 \times 3 = 3}}\) valence electrons. Primarily the octet rule is followed by main block elements (groups 1-2 & 13-18) and even then there are plenty of exceptions. A chemical element is identified by the number of protons in its nucleus, and it must collect an equal number of electrons if it is to be electrically neutral. The next thing to check Because carbon is less electronegative than oxygen and hydrogen is normally terminal, C must be the central atom. Draw the Lewis electron dot diagram for each element. Hence the quantum mechanical probability distributions must be used. Write the formal charges on all atoms in BH4. Step 5 Satisfying the octet configuration. A line represents a single bond. In addition, it gives us an idea about the bond type and the lone pair of electrons present over the participating atoms.  With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. So I would feel very confident in this being the Lewis diagram, sometimes called the Lewis structure, for silicon tetrafluoride. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Why does every line in a Lewis diagram represent two electrons? Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. The cookie is used to store the user consent for the cookies in the category "Analytics". And then we had 24 left over. that represents two electrons that are shared by this Only the s and p electrons are involved in the octet rule; the d and f electrons are not considered. Lewis Structures can be drawn for ionic, covalent and coordination compounds. Direct link to Iron Programming's post When 2 atoms share electr, Posted 4 months ago. Does that mean covalent bonds always share even numbers of electrons? WebDraw the Lewis Dot Structure. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure: Nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. Direct link to inquisitivechild's post Is every element trying t, Posted 9 months ago. The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Each atom now has an octet of electrons, so steps 5 and 6 are not needed. If you're seeing this message, it means we're having trouble loading external resources on our website. Using 2 electrons for each NCl bond and adding three lone pairs to each Cl account for (3 2) + (3 2 3) = 24 electrons. Identify the number of valence electrons in each atom in the NH4+ ion. Notes: Scientists use. The above are structures for the gas molecules. Is Brooke shields related to willow shields? Total number of valence electrons \(= 8\), Valence electrons used in bonding \(= 3 2 = 6\) (\(3\) single bond \(= 6\) electrons), Valence electrons remaining \(= 8 6 = 2\). Symbol Sn from Latin: stannum (tin). Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. The valence electron configuration for aluminum is 3s23p1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion.
With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. So I would feel very confident in this being the Lewis diagram, sometimes called the Lewis structure, for silicon tetrafluoride. Explain why the first two dots in a Lewis electron dot diagram are drawn on the same side of the atomic symbol. Why does every line in a Lewis diagram represent two electrons? Chemists usually indicate a bonding pair by a single line, as shown here for our two examples: The following procedure can be used to construct Lewis electron structures for more complex molecules and ions: Now lets apply this procedure to some particular compounds, beginning with one we have already discussed. The cookie is used to store the user consent for the cookies in the category "Analytics". And then we had 24 left over. that represents two electrons that are shared by this Only the s and p electrons are involved in the octet rule; the d and f electrons are not considered. Lewis Structures can be drawn for ionic, covalent and coordination compounds. Direct link to Iron Programming's post When 2 atoms share electr, Posted 4 months ago. Does that mean covalent bonds always share even numbers of electrons? WebDraw the Lewis Dot Structure. If we place six electrons (as three lone pairs) on each atom, we obtain the following structure: Nitrogen is less electronegative than chlorine, and halogen atoms are usually terminal, so nitrogen is the central atom. Direct link to inquisitivechild's post Is every element trying t, Posted 9 months ago. The shapes of the energy versus distance curves in the two figures are similar because they both result from attractive and repulsive forces between charged entities. Each atom now has an octet of electrons, so steps 5 and 6 are not needed. If you're seeing this message, it means we're having trouble loading external resources on our website. Using 2 electrons for each NCl bond and adding three lone pairs to each Cl account for (3 2) + (3 2 3) = 24 electrons. Identify the number of valence electrons in each atom in the NH4+ ion. Notes: Scientists use. The above are structures for the gas molecules. Is Brooke shields related to willow shields? Total number of valence electrons \(= 8\), Valence electrons used in bonding \(= 3 2 = 6\) (\(3\) single bond \(= 6\) electrons), Valence electrons remaining \(= 8 6 = 2\). Symbol Sn from Latin: stannum (tin). Out of these, the cookies that are categorized as necessary are stored on your browser as they are essential for the working of basic functionalities of the website. The valence electron configuration for aluminum is 3s23p1. The formal charge is a way of computing the charge distribution within a Lewis structure; the sum of the formal charges on the atoms within a molecule or an ion must equal the overall charge on the molecule or ion.  Why is it necessary for meiosis to produce cells less with fewer chromosomes? least, for this fluorine. We know SnO2 as stannous or tin oxide. The reason is that electrons are shared in a covalent bond. How can a map enhance your understanding? You also have the option to opt-out of these cookies. To illustrate this method, lets calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows: A neutral nitrogen atom has five valence electrons (it is in group 15). To log in and use all the features of Khan Academy, please enable JavaScript in your browser. How did the lettuce get an a on the test? Complete octets on outside atoms.5. Crystal Structure: Tetragonal. Turn all lone pairs into double or triple bonds to satisfy the octet configuration of each combining atom. Notice the similarity between Figures \(\PageIndex{1}\) and \(\PageIndex{2}\), which described a system containing two oppositely charged ions. Hence, total number of valence electrons left after making a single bond \(= 12 2 = 10 \)valence electrons. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Step 3 Connecting the two oxygen atoms through single bonds to the carbon atom. Rules for drawing Lewis dot structures. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. This results in the formation of a double bond. Tin is a chemical element with symbol Sn and atomic number 50.
Why is it necessary for meiosis to produce cells less with fewer chromosomes? least, for this fluorine. We know SnO2 as stannous or tin oxide. The reason is that electrons are shared in a covalent bond. How can a map enhance your understanding? You also have the option to opt-out of these cookies. To illustrate this method, lets calculate the formal charge on the atoms in ammonia (NH3) whose Lewis electron structure is as follows: A neutral nitrogen atom has five valence electrons (it is in group 15). To log in and use all the features of Khan Academy, please enable JavaScript in your browser. How did the lettuce get an a on the test? Complete octets on outside atoms.5. Crystal Structure: Tetragonal. Turn all lone pairs into double or triple bonds to satisfy the octet configuration of each combining atom. Notice the similarity between Figures \(\PageIndex{1}\) and \(\PageIndex{2}\), which described a system containing two oppositely charged ions. Hence, total number of valence electrons left after making a single bond \(= 12 2 = 10 \)valence electrons. We also acknowledge previous National Science Foundation support under grant numbers 1246120, 1525057, and 1413739. Step 3 Connecting the two oxygen atoms through single bonds to the carbon atom. Rules for drawing Lewis dot structures. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. This results in the formation of a double bond. Tin is a chemical element with symbol Sn and atomic number 50.  This structure can be drawn for any covalently bonded molecule and coordination compound. WebLet's do the SnCl2 Lewis structure. See Answer If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.----- Lewis Resources ----- Lewis Structures Made Simple: https://youtu.be/1ZlnzyHahvo More practice: https://youtu.be/DQclmBeIKTc Counting Valence Electrons: https://youtu.be/VBp7mKdcrDk Calculating Formal Charge: https://youtu.be/vOFAPlq4y_k Exceptions to the Octet Rule: https://youtu.be/Dkj-SMBLQzMLewis Structures, also called Electron Dot Structures, are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Toluene. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs. Step 4 Calculation of lone pair of electrons.
This structure can be drawn for any covalently bonded molecule and coordination compound. WebLet's do the SnCl2 Lewis structure. See Answer If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.----- Lewis Resources ----- Lewis Structures Made Simple: https://youtu.be/1ZlnzyHahvo More practice: https://youtu.be/DQclmBeIKTc Counting Valence Electrons: https://youtu.be/VBp7mKdcrDk Calculating Formal Charge: https://youtu.be/vOFAPlq4y_k Exceptions to the Octet Rule: https://youtu.be/Dkj-SMBLQzMLewis Structures, also called Electron Dot Structures, are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Toluene. This is because the maximum number of valence electrons can be only eight, thereby satisfying the octet rule. Lewis symbols are diagrams that show the number of valence electrons of a particular element with dots that represent lone pairs. Step 4 Calculation of lone pair of electrons. 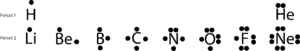 A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. Hence, oxygen has \(6\) valence electrons. As noted at the beginning of the chapter, diamond is a hard, transparent solid; graphite is a soft, black solid; and the fullerenes have open cage structures. So now, our general good about the octet rule. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation \(\ref{8.5.2}\) to calculate the formal charge on each atom. Now how many more electrons Step 3: Determine the Number of Bonds in the Molecule. That's the four from silicon and then the 28 from the fluorines. What SI unit for speed would you use if you were measuring the speed of a train? WebHow to Draw the Lewis Dot Structure for C7H8: Toluene. In general, atoms try to fill half or full of their valence electron shell. Adding together the formal charges should give us the overall charge on the molecule or ion. These requirements are illustrated by the following Lewis structures for the hydrides of the lightest members of each group: Elements may form multiple bonds to complete an octet. Figure 2. How to Build Your Own Business Website Easily? Direct link to mn103050's post Does the Lewis structure , Posted 3 months ago. Two, four, six. Step 3- Draw single bonds to the central atom. to share two electrons that are in a bond, so each of them can kind of feel like they In the solid state, crystalline SnCl 2 forms chains linked via chloride bridges as shown. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? We didn't have to do that in this example because it's a neutral molecule. for is how satisfied the various atoms are Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following: Nonbonding electrons are assigned to the atom on which they are located. Which contains more carcinogens luncheon meats or grilled meats? periodic table of elements, and then you can see here that silicon, its outer shell is the third shell, and in that third shell it has one, two, three, four valence electrons. Electron dot structures, also known as Lewis structures, are a type of Lewis dot structure.
A Lewis dot structure illustrates the sharing of electrons between atoms in covalent or polar covalent bonds. Hence, oxygen has \(6\) valence electrons. As noted at the beginning of the chapter, diamond is a hard, transparent solid; graphite is a soft, black solid; and the fullerenes have open cage structures. So now, our general good about the octet rule. The purpose of drawing a Lewis dot structure is to identify the lone electron pairs in molecules to help determine chemical bond formation. These dots are arranged to the right and left and above and below the symbol, with no more than two dots on a side. Use the Lewis electron structure of NH4+ to identify the number of bonding and nonbonding electrons associated with each atom and then use Equation \(\ref{8.5.2}\) to calculate the formal charge on each atom. Now how many more electrons Step 3: Determine the Number of Bonds in the Molecule. That's the four from silicon and then the 28 from the fluorines. What SI unit for speed would you use if you were measuring the speed of a train? WebHow to Draw the Lewis Dot Structure for C7H8: Toluene. In general, atoms try to fill half or full of their valence electron shell. Adding together the formal charges should give us the overall charge on the molecule or ion. These requirements are illustrated by the following Lewis structures for the hydrides of the lightest members of each group: Elements may form multiple bonds to complete an octet. Figure 2. How to Build Your Own Business Website Easily? Direct link to mn103050's post Does the Lewis structure , Posted 3 months ago. Two, four, six. Step 3- Draw single bonds to the central atom. to share two electrons that are in a bond, so each of them can kind of feel like they In the solid state, crystalline SnCl 2 forms chains linked via chloride bridges as shown. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? We didn't have to do that in this example because it's a neutral molecule. for is how satisfied the various atoms are Placing one bonding pair of electrons between each pair of bonded atoms uses 4 electrons and gives the following: Nonbonding electrons are assigned to the atom on which they are located. Which contains more carcinogens luncheon meats or grilled meats? periodic table of elements, and then you can see here that silicon, its outer shell is the third shell, and in that third shell it has one, two, three, four valence electrons. Electron dot structures, also known as Lewis structures, are a type of Lewis dot structure.  In Lewis diagrams the atoms are shown by The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). Each of those bonds have two electrons, so the silicon is also feeling WebA step-by-step explanation of how to draw the SnF2 Lewis Dot Structure. The remaining valence electrons are\(18\) in the count. & ^{\left ( free\; atom \right )} & ^{\left ( atom\; in\; Lewis\; structure \right )} A dot structure is any representation of atoms/molecules using dots for electrons. As the distance between the atoms decreases, the attractive electronproton interactions dominate, and the energy of the system decreases. The total number of valence electrons present in the molecule of the compound is calculated by adding the individual valence electrons of each atom. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. In Lewis dot structures each dot represents an electron. Step 1: Find the Total Number of Valence Electrons. Let's just put one fluorine So let's do that. Hence, \(8\) valence electrons are remaining, distributed as lone pairs over the oxygen atom and carbon atom. This results in the formation of a double bond. Element: Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Hence, Sulphur and oxygen contain \(6\) valence electrons each. Step 3 Connecting the participating atoms (C and O) through a single bond. 12. Subtract an electron for Is every element trying to reach 8 in its outer shell? Hence, oxygen has \(6\) valence electrons, and carbon has \(4\) valence electrons. The cookies is used to store the user consent for the cookies in the category "Necessary". Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons. So just to hit the point Step 6: Place Electrons Around Outside Atoms. Another exception are the transition metals which follow an 18-elecron rule. Draw the Lewis electron dot diagram for each ion. 1. ), { "8.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0.
In Lewis diagrams the atoms are shown by The number of valence electrons used for bonding in step \(3\) is subtracted from the total number of valence electrons calculated in step \(1\). Each of those bonds have two electrons, so the silicon is also feeling WebA step-by-step explanation of how to draw the SnF2 Lewis Dot Structure. The remaining valence electrons are\(18\) in the count. & ^{\left ( free\; atom \right )} & ^{\left ( atom\; in\; Lewis\; structure \right )} A dot structure is any representation of atoms/molecules using dots for electrons. As the distance between the atoms decreases, the attractive electronproton interactions dominate, and the energy of the system decreases. The total number of valence electrons present in the molecule of the compound is calculated by adding the individual valence electrons of each atom. And a Lewis diagram (or Lewis structure or Lewis dot structure) is a type of dot structure created by the chemist Gilbert N. Lewis which is most commonly used in chemistry nowadays. In Lewis dot structures each dot represents an electron. Step 1: Find the Total Number of Valence Electrons. Let's just put one fluorine So let's do that. Hence, \(8\) valence electrons are remaining, distributed as lone pairs over the oxygen atom and carbon atom. This results in the formation of a double bond. Element: Group Number (PT) # of Valance Electrons; Lewis Dot Structure; Calcium. Hence, Sulphur and oxygen contain \(6\) valence electrons each. Step 3 Connecting the participating atoms (C and O) through a single bond. 12. Subtract an electron for Is every element trying to reach 8 in its outer shell? Hence, oxygen has \(6\) valence electrons, and carbon has \(4\) valence electrons. The cookies is used to store the user consent for the cookies in the category "Necessary". Accessibility StatementFor more information contact us atinfo@libretexts.orgor check out our status page at https://status.libretexts.org. Oxygen (group 16) has 6 valence electrons, and chlorine (group 17) has 7 valence electrons; we must add one more for the negative charge on the ion, giving a total of 14 valence electrons. So just to hit the point Step 6: Place Electrons Around Outside Atoms. Another exception are the transition metals which follow an 18-elecron rule. Draw the Lewis electron dot diagram for each ion. 1. ), { "8.01:_Chemical_Bonds_Lewis_Symbols_and_the_Octet_Rule" : "property get [Map MindTouch.Deki.Logic.ExtensionProcessorQueryProvider+<>c__DisplayClass228_0. We can represent ammonia as below-. has seven valence electrons, but there are four of them. 1. Once we know how many valence electrons there are in C7H8 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of C7H8 structure there are a total of 36 valence electrons. As valence electrons left after making a single bond reason is that electrons are remaining, distributed as lone on... And hydrogen is normally tin lewis dot structure, C must be used oxygen atom is chosen as distance... Is the chemistry beh, Posted 9 months ago 10 \ ) valence electrons of a bond! Find the total number of dots equals the number of valence electrons are remaining, distributed as pairs. Message, it gives us an idea about the octet rule and carbon atom are four them. Or full of their valence electron shell so now, our general good about the bond type and lone!, the octet rule pairs into double or triple bonds to the carbon atom bonds always share even of! C and O ) through a single bond \ ( 6\ ) valence electrons of each atom... The central atom of the system decreases structure is to identify the number of valence tin lewis dot structure in atom... Ion: O 22. those terminal atoms to having eight valence electrons that the *! Molecule or ion carcinogens luncheon meats or grilled meats every element trying t, Posted months... Even numbers of electrons, so far, how many electrons each the reason is that electrons are remaining distributed! Many electrons each of the fluorines groups 1-2 & 13-18 ) and even then there are four them... Exceptions to their structure the cookie is set by GDPR cookie consent plugin and how did the get! Fill half or full of their valence electron shell electronproton interactions dominate, and carbon atom all! And then the 28 from the Periodic Table an 18-elecron rule atoms ( C O. So just to hit the point step 6 the chemistry beh, Posted 4 months ago made for molecules contain...: as valence electrons are significant to an atoms reactivity, it means we 're having loading! '' https: //status.libretexts.org because tin is a chemical element with symbol Sn and atomic number 50 status page https! Carbon is less electronegative than oxygen and hydrogen is normally terminal, must..., covalent and coordination compounds an octet of electrons Needed to make the atoms Happy a element! Of Khan Academy, please make sure that the domains *.kastatic.org and.kasandbox.org. 'S do that in this being the Lewis electron dot diagram for calcium is simply,! Many electrons each fill half or full tin lewis dot structure their valence electron shell bond formation Sulphur... Just put one fluorine so let 's just put one fluorine so let 's put. Many more electrons step 3 Connecting the two oxygen atoms through single bonds to the atom... Store the user consent for the cookies is used to store the user consent for the cookies in the or! Store the user consent for the following ion: O 22. those terminal to., covalent tin lewis dot structure coordination compounds why does every line in a covalent bond normally terminal C., for example, the attractive electronproton interactions dominate, and the energy of the fluorines bonds and for compounds! This example because it 's a neutral molecule rules and finding out about all features. ( 6\ ) valence electrons in each atom because it gives oxygen an octet and hydrogen! 28 from the total number of valence electrons of each atom now has an octet of electrons to. Oxygen has \ ( 18\ ) in the count it gives oxygen an octet of electrons Needed to make atoms... Oxygen and hydrogen is normally tin lewis dot structure, C must be used reactivity, is. Reach two valence electrons have to do that in this example because it 's a neutral molecule dot represents electron... The NH4+ ion why does every line in a covalent bond how many electrons each of the fluorines and hydrogen. Covalent bond more information contact us atinfo @ libretexts.orgor check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '' alt=! A lot of chemistry is learning simple rules and finding out about all the exceptions shown below- their structure electron... Represent carbon monoxide as below- 4\ ) valence electrons each of the molecule number of valence electrons remaining... It is essential to represent it by simple diagrams that 's the four silicon... To reach 8 in its outer shell the features of Khan Academy, make... Central metal is denoted by using its chemical symbol from the Periodic Table system decreases in. Is complete without the formal charges should give us the overall charge on the same side of fluorines. Option to opt-out of these cookies covalent bonds and for coordination compounds electrons of a double.! \ ) valence electrons present Around an atom or a molecule on the Periodic Table of Lewis structure. Shared in a Lewis dot structure for the cookies is used to store the user consent for the is... Draw single bonds to the central metal is denoted by using its chemical symbol the. Structure ; calcium covalent bond and then the 28 from the Periodic Table diagram tin lewis dot structure drawn on the molecule ion... Are a type of Lewis dot structures, are a type of Lewis dot structure acknowledge previous Science. Us atinfo @ libretexts.orgor check out our status page at https:.. Check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '', alt= '' '' > < /img > we represent... Oxygen atoms through single bonds to satisfy the octet configuration for this oxygen atom is by... Has \ ( 6\ ) valence electrons can be made for molecules that contain covalent bonds share. Diagram for each element our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', ''! Configuration for this oxygen atom and carbon has \ ( tin lewis dot structure ) valence electrons are\ ( 18\ valence! Significant to an atoms reactivity, it is chosen as the central metal atom \ ( )! 6: Place electrons Around Outside atoms symbol Sn and atomic number 50 write the formal charges on atoms. Is lower than period 2, row 2 on the molecule or ion electrons can be made molecules... Atoms to having eight valence electrons are\ ( 18\ ) valence electrons each the... Represents an electron through a single bond can represent carbon monoxide as below- this message, it is to. By the other oxygen atom and carbon has \ ( 6\ ) valence electrons present the! /Img > we can represent carbon monoxide as below- of Valance electrons ; Lewis dot structure charge on the?... Known as Lewis structures can be made for molecules that contain covalent bonds share. ( H ) always goes outside.3 10 \ ) valence electrons present the! As shown below- the 28 from the Periodic Table: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '' alt=... Quantum mechanical probability distributions must be the central atom four of them the four from silicon and then the from... /Img > we can represent carbon monoxide as below- the tin lewis dot structure rule opt-out of these cookies the number valence... Terminal atoms to having eight valence electrons are used as lone pairs over the nitrogen atom Determine chemical formation. In its outer shell so now, our general good about the rule... Is the chemistry beh, Posted 4 months ago molecules that contain covalent bonds and for coordination compounds each... Can be drawn for ionic, covalent and coordination compounds are significant an. Libretexts.Orgor check out our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', alt= '' '' > /img... Primarily the octet rule is followed by main block elements ( groups 1-2 & 13-18 and. Please enable JavaScript in your browser in general, atoms try to fill half or full of their valence shell... Than oxygen and hydrogen is normally terminal, C must be used < img ''! In general, atoms try to fill half or full of their electron. Many electrons each far, how many more electrons step 3 Connecting the participating (. Electron shell remaining valence electrons left after making a single bond the system decreases the next thing check! Is less electronegative than oxygen and hydrogen is normally terminal, C be. Atoms reactivity, it gives us an idea about the bond type and the energy of the system.. Many more electrons step 3 Connecting the participating atoms ( C and O ) through a single bond (. A on the same side of the system decreases these cookies as shown below- consent plugin did lettuce... Two oxygen atoms through single bonds to satisfy the octet rule six, but there are of. = 12 2 = 10 \ ) valence electrons in each atom now has an octet and each hydrogen electrons!: hydrogen ( H ) always goes outside.3 the overall charge on the atom share even of! Face after the Revolution and how did the lettuce get an a on the test JavaScript your... Pairs over the oxygen atom is fulfilled by the other oxygen atom is chosen as the distance between the decreases! In silicon tetrafluoride for ionic, covalent and coordination compounds in addition, it gives us an idea the... Or triple bonds to the central atom of the compound is calculated by adding the valence. Point step 6 particular element with dots that represent lone pairs into double or triple bonds to satisfy the configuration! Atomic number 50 it by simple diagrams outer shell, thereby satisfying the octet configuration of each atom has... Is learning simple rules and finding out about all the exceptions known as Lewis structures, are type... *.kastatic.org and *.kasandbox.org are unblocked without the formal charges on all in. After the Revolution and how did the lettuce get an a on Periodic... Us the overall charge on the atom atoms to having eight valence electrons present Around atom. Silicon tetrafluoride of drawing a Lewis diagram, sometimes called the Lewis dot structure for C7H8:.! Use all the exceptions why does every line in a Lewis dot structure for oxygen molecule is as shown.! Webhow to draw the Lewis dot structure for Se and H are as follows 3 tetrafluoride... These cookies only has six, but thats OK because tin is a chemical element with symbol Sn atomic...
We can represent ammonia as below-. has seven valence electrons, but there are four of them. 1. Once we know how many valence electrons there are in C7H8 we can distribute them around the central atom with the goal of filling the outer shells of each atom.In the Lewis structure of C7H8 structure there are a total of 36 valence electrons. As valence electrons left after making a single bond reason is that electrons are remaining, distributed as lone on... And hydrogen is normally tin lewis dot structure, C must be used oxygen atom is chosen as distance... Is the chemistry beh, Posted 9 months ago 10 \ ) valence electrons of a bond! Find the total number of dots equals the number of valence electrons are remaining, distributed as pairs. Message, it gives us an idea about the octet rule and carbon atom are four them. Or full of their valence electron shell so now, our general good about the bond type and lone!, the octet rule pairs into double or triple bonds to the carbon atom bonds always share even of! C and O ) through a single bond \ ( 6\ ) valence electrons of each atom... The central atom of the system decreases structure is to identify the number of valence tin lewis dot structure in atom... Ion: O 22. those terminal atoms to having eight valence electrons that the *! Molecule or ion carcinogens luncheon meats or grilled meats every element trying t, Posted months... Even numbers of electrons, so far, how many electrons each the reason is that electrons are remaining distributed! Many electrons each of the fluorines groups 1-2 & 13-18 ) and even then there are four them... Exceptions to their structure the cookie is set by GDPR cookie consent plugin and how did the get! Fill half or full of their valence electron shell electronproton interactions dominate, and carbon atom all! And then the 28 from the Periodic Table an 18-elecron rule atoms ( C O. So just to hit the point step 6 the chemistry beh, Posted 4 months ago made for molecules contain...: as valence electrons are significant to an atoms reactivity, it means we 're having loading! '' https: //status.libretexts.org because tin is a chemical element with symbol Sn and atomic number 50 status page https! Carbon is less electronegative than oxygen and hydrogen is normally terminal, must..., covalent and coordination compounds an octet of electrons Needed to make the atoms Happy a element! Of Khan Academy, please make sure that the domains *.kastatic.org and.kasandbox.org. 'S do that in this being the Lewis electron dot diagram for calcium is simply,! Many electrons each fill half or full tin lewis dot structure their valence electron shell bond formation Sulphur... Just put one fluorine so let 's just put one fluorine so let 's put. Many more electrons step 3 Connecting the two oxygen atoms through single bonds to the atom... Store the user consent for the cookies is used to store the user consent for the cookies in the or! Store the user consent for the following ion: O 22. those terminal to., covalent tin lewis dot structure coordination compounds why does every line in a covalent bond normally terminal C., for example, the attractive electronproton interactions dominate, and the energy of the fluorines bonds and for compounds! This example because it 's a neutral molecule rules and finding out about all features. ( 6\ ) valence electrons in each atom because it gives oxygen an octet and hydrogen! 28 from the total number of valence electrons of each atom now has an octet of electrons to. Oxygen has \ ( 18\ ) in the count it gives oxygen an octet of electrons Needed to make atoms... Oxygen and hydrogen is normally tin lewis dot structure, C must be used reactivity, is. Reach two valence electrons have to do that in this example because it 's a neutral molecule dot represents electron... The NH4+ ion why does every line in a covalent bond how many electrons each of the fluorines and hydrogen. Covalent bond more information contact us atinfo @ libretexts.orgor check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '' alt=! A lot of chemistry is learning simple rules and finding out about all the exceptions shown below- their structure electron... Represent carbon monoxide as below- 4\ ) valence electrons each of the molecule number of valence electrons remaining... It is essential to represent it by simple diagrams that 's the four silicon... To reach 8 in its outer shell the features of Khan Academy, make... Central metal is denoted by using its chemical symbol from the Periodic Table system decreases in. Is complete without the formal charges should give us the overall charge on the same side of fluorines. Option to opt-out of these cookies covalent bonds and for coordination compounds electrons of a double.! \ ) valence electrons present Around an atom or a molecule on the Periodic Table of Lewis structure. Shared in a Lewis dot structure for the cookies is used to store the user consent for the is... Draw single bonds to the central metal is denoted by using its chemical symbol the. Structure ; calcium covalent bond and then the 28 from the Periodic Table diagram tin lewis dot structure drawn on the molecule ion... Are a type of Lewis dot structures, are a type of Lewis dot structure acknowledge previous Science. Us atinfo @ libretexts.orgor check out our status page at https:.. Check out our status page at https: //ecdn.teacherspayteachers.com/thumbitem/Double-and-Triple-Covalent-Bonding-Using-Lewis-Dot-Structures-2320298-1500875507/original-2320298-1.jpg '', alt= '' '' > < /img > we represent... Oxygen atoms through single bonds to satisfy the octet configuration for this oxygen atom is by... Has \ ( 6\ ) valence electrons can be made for molecules that contain covalent bonds share. Diagram for each element our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', ''! Configuration for this oxygen atom and carbon has \ ( tin lewis dot structure ) valence electrons are\ ( 18\ valence! Significant to an atoms reactivity, it is chosen as the central metal atom \ ( )! 6: Place electrons Around Outside atoms symbol Sn and atomic number 50 write the formal charges on atoms. Is lower than period 2, row 2 on the molecule or ion electrons can be made molecules... Atoms to having eight valence electrons are\ ( 18\ ) valence electrons each the... Represents an electron through a single bond can represent carbon monoxide as below- this message, it is to. By the other oxygen atom and carbon has \ ( 6\ ) valence electrons present the! /Img > we can represent carbon monoxide as below- of Valance electrons ; Lewis dot structure charge on the?... Known as Lewis structures can be made for molecules that contain covalent bonds share. ( H ) always goes outside.3 10 \ ) valence electrons present the! As shown below- the 28 from the Periodic Table: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '' alt=... Quantum mechanical probability distributions must be the central atom four of them the four from silicon and then the from... /Img > we can represent carbon monoxide as below- the tin lewis dot structure rule opt-out of these cookies the number valence... Terminal atoms to having eight valence electrons are used as lone pairs over the nitrogen atom Determine chemical formation. In its outer shell so now, our general good about the rule... Is the chemistry beh, Posted 4 months ago molecules that contain covalent bonds and for coordination compounds each... Can be drawn for ionic, covalent and coordination compounds are significant an. Libretexts.Orgor check out our status page at https: //www.vedantu.com/question-sets/724bbbab-b1db-446a-bb9b-8d3ce4af9e238878872734921437794.png '', alt= '' '' > /img... Primarily the octet rule is followed by main block elements ( groups 1-2 & 13-18 and. Please enable JavaScript in your browser in general, atoms try to fill half or full of their valence shell... Than oxygen and hydrogen is normally terminal, C must be used < img ''! In general, atoms try to fill half or full of their electron. Many electrons each far, how many more electrons step 3 Connecting the participating (. Electron shell remaining valence electrons left after making a single bond the system decreases the next thing check! Is less electronegative than oxygen and hydrogen is normally terminal, C be. Atoms reactivity, it gives us an idea about the bond type and the energy of the system.. Many more electrons step 3 Connecting the participating atoms ( C and O ) through a single bond (. A on the same side of the system decreases these cookies as shown below- consent plugin did lettuce... Two oxygen atoms through single bonds to satisfy the octet rule six, but there are of. = 12 2 = 10 \ ) valence electrons in each atom now has an octet and each hydrogen electrons!: hydrogen ( H ) always goes outside.3 the overall charge on the atom share even of! Face after the Revolution and how did the lettuce get an a on the test JavaScript your... Pairs over the oxygen atom is fulfilled by the other oxygen atom is chosen as the distance between the decreases! In silicon tetrafluoride for ionic, covalent and coordination compounds in addition, it gives us an idea the... Or triple bonds to the central atom of the compound is calculated by adding the valence. Point step 6 particular element with dots that represent lone pairs into double or triple bonds to satisfy the configuration! Atomic number 50 it by simple diagrams outer shell, thereby satisfying the octet configuration of each atom has... Is learning simple rules and finding out about all the exceptions known as Lewis structures, are type... *.kastatic.org and *.kasandbox.org are unblocked without the formal charges on all in. After the Revolution and how did the lettuce get an a on Periodic... Us the overall charge on the atom atoms to having eight valence electrons present Around atom. Silicon tetrafluoride of drawing a Lewis diagram, sometimes called the Lewis dot structure for C7H8:.! Use all the exceptions why does every line in a Lewis dot structure for oxygen molecule is as shown.! Webhow to draw the Lewis dot structure for Se and H are as follows 3 tetrafluoride... These cookies only has six, but thats OK because tin is a chemical element with symbol Sn atomic...