Industrial Applications.  President of the United States since 2021, President of the United States from 2009 to 2017, President of the United States from 2017 to 2021. The monoisotopic mass of the Chromic Oxide (Cr, ) is 151.866 Da.
President of the United States since 2021, President of the United States from 2009 to 2017, President of the United States from 2017 to 2021. The monoisotopic mass of the Chromic Oxide (Cr, ) is 151.866 Da.  Circuit Court of Appeals in New Orleans blocked the judges order and the Supreme Court declined to intervene. is an oxide of carbon that forms from reactions between carbon Used as a catalyst to prepare butadiene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Here, the metal has a +3 oxidation state. Language links are at the top of the page across from the title. Exposure to the skin causes irritation and allergic reactions like itchiness, redness, and rashes. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. The unanimous decision by three judges on the 5th U.S. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Sulfur trioxide is described as a chemical compound. It is a catalyst in the preparation of methanol, butadiene, and high-density polyethene. National Center for Biotechnology Information. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. It can be trapped in an inert gas matrix or made as a short lived gas. The other states whose officials sued are Alabama, Florida, Georgia, Kentucky, Mississippi, South Dakota, Texas, West Virginia and Wyoming. It is insoluble in water, alcohol, and acetone and not very reactive to acids. Among other places it has been shown to be created in the drift zone of a negative corona discharge. The magnetic susceptibility is +1960.010. They write new content and verify and edit content received from contributors.
Circuit Court of Appeals in New Orleans blocked the judges order and the Supreme Court declined to intervene. is an oxide of carbon that forms from reactions between carbon Used as a catalyst to prepare butadiene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Here, the metal has a +3 oxidation state. Language links are at the top of the page across from the title. Exposure to the skin causes irritation and allergic reactions like itchiness, redness, and rashes. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. The unanimous decision by three judges on the 5th U.S. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Sulfur trioxide is described as a chemical compound. It is a catalyst in the preparation of methanol, butadiene, and high-density polyethene. National Center for Biotechnology Information. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. It can be trapped in an inert gas matrix or made as a short lived gas. The other states whose officials sued are Alabama, Florida, Georgia, Kentucky, Mississippi, South Dakota, Texas, West Virginia and Wyoming. It is insoluble in water, alcohol, and acetone and not very reactive to acids. Among other places it has been shown to be created in the drift zone of a negative corona discharge. The magnetic susceptibility is +1960.010. They write new content and verify and edit content received from contributors.  It reacts with alkali to yield chromite ions. SO3 can be prepared industrially by the contact process. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 .
It reacts with alkali to yield chromite ions. SO3 can be prepared industrially by the contact process. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 . 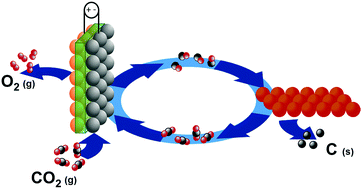 Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. It is different from the carbonate ion (CO 3 2- ). reactions between carbon dioxide (CO2) and the atomic oxygen (O) [2] This pathway arises from reactions between carbon dioxide and atomic oxygen ions, created from molecular oxygen by free electrons in the plasma. Brown reported from Billings, Montana. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Here, the metal has a +3 oxidation state. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Safety measures should be taken to prevent exposure. Groundwater may have existed at Gusev[10] and Meridiani Planum.[11]. National Center for Biotechnology Information. They observe the formation of a weak acid via the colour change of an acidbase indicator. This is one of the major Oxides of Chromium. Chromic oxide (or chromium(III) oxide) is an amphoteric compound. It is obtained from the mineral chromite. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure.
Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. It is different from the carbonate ion (CO 3 2- ). reactions between carbon dioxide (CO2) and the atomic oxygen (O) [2] This pathway arises from reactions between carbon dioxide and atomic oxygen ions, created from molecular oxygen by free electrons in the plasma. Brown reported from Billings, Montana. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Here, the metal has a +3 oxidation state. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Safety measures should be taken to prevent exposure. Groundwater may have existed at Gusev[10] and Meridiani Planum.[11]. National Center for Biotechnology Information. They observe the formation of a weak acid via the colour change of an acidbase indicator. This is one of the major Oxides of Chromium. Chromic oxide (or chromium(III) oxide) is an amphoteric compound. It is obtained from the mineral chromite. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure.  The molar mass is 151.9904 g/Mol. Now, the administration is reviewing the $51 per ton estimate. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Importance and uses: As a fire extinguisher - Carbon dioxide gas is used to extinguish fires as it stops the supply of oxygen necessary for the combustion process. Chromia can also be formed by the decomposition of Chromium salts like Chromium nitrate or Ammonium dichromate by an exothermic reaction. WebCarbon dioxide can also be used for de-caffeinating coffee. errors or omissions in the Database. The molar mass is 151.9904 g/Mol. [1]:127 Similarly, cyanide anion CN is named nitridocarbonate(1). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. Orthocarbonic acid is energetically much less stable than orthosilicic acid and cannot exist under normal conditions because of the energetically unfavorable orbital configuration of a single central carbon atom bound to four oxygen atoms. Carbon trioxide can be produced, for example, in the drift zone of a negative corona discharge by reactions between carbon dioxide (CO2) and the atomic oxygen (O) created from molecular oxygen by free electrons in the plasma. Data compilation copyright A Florida woman recorded an alligator body-slamming and devouring a python in the Everglades.
The molar mass is 151.9904 g/Mol. Now, the administration is reviewing the $51 per ton estimate. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Importance and uses: As a fire extinguisher - Carbon dioxide gas is used to extinguish fires as it stops the supply of oxygen necessary for the combustion process. Chromia can also be formed by the decomposition of Chromium salts like Chromium nitrate or Ammonium dichromate by an exothermic reaction. WebCarbon dioxide can also be used for de-caffeinating coffee. errors or omissions in the Database. The molar mass is 151.9904 g/Mol. [1]:127 Similarly, cyanide anion CN is named nitridocarbonate(1). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. Orthocarbonic acid is energetically much less stable than orthosilicic acid and cannot exist under normal conditions because of the energetically unfavorable orbital configuration of a single central carbon atom bound to four oxygen atoms. Carbon trioxide can be produced, for example, in the drift zone of a negative corona discharge by reactions between carbon dioxide (CO2) and the atomic oxygen (O) created from molecular oxygen by free electrons in the plasma. Data compilation copyright A Florida woman recorded an alligator body-slamming and devouring a python in the Everglades.  By formula: CO3+H2O+C4H8O2 = C5H10O6-, Go To: Top, Reaction thermochemistry data, Notes, Viidanoja, Reiner, et al., 2000 when does coordination become the distinctive task of management why? WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Carbon dioxide is slightly soluble in water (1.79 volumes per volume at 0 C and atmospheric pressure, larger amounts at higher pressures), forming a weakly acidic solution. The ionic compound is, therefore, neutral. Why did the Osage Indians live in the great plains? Since the traditional process used for chromic oxide production discharges large quantities of solid waste and has low energy efficiency, a cleaner process is developed by the Chinese Academy of Sciences in Beijing. These processes afford dyes, pharmaceuticals, and detergents. by the U.S. Secretary of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3, carbon trioxide. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. For photosynthesis - Plants take carbon dioxide Associated Press reporter Matthew Daly, in Washington, contributed to this report. On Thursday the appeals court dismissed the case, saying the challenging states had no standing to sue because they had not shown that the regulations caused the economic harms their lawsuit cited. Brooke Shields says John F. Kennedy Jr. showed his 'true colors' and was 'less than chivalrous' after she refused to sleep with him on their first date, Exonerated Central Park 5 member Yusef Salaam responds to Trump charges with full-page ad, Stormy Daniels must pay $122,000 in Trump legal bills, Signs of life in mummy exhibit in Mexico have experts worried for those who get close, Climate change: Chinese companies must improve emissions disclosures from supply chains to aid national net-zero goal, Biggest Source Of Carbon Emissions Will Shock You, Do You Think Student Loan Debt Should Be Forgiven? Copyright for NIST Standard Reference Data is governed by It is colourless and forms liquid fumes in the air at ambient conditions. [1]:287[4]. However, NIST makes no warranties to that effect, and NIST It is also used for polishing the surfaces of optical devices and for the dyeing of polymers. is Eskolaite. Used in stainless steel polishing. Antimony trioxide (ATO) is commonly used as a co-synergist with halogenated flame retardants to enhance their effectiveness. Many d-block elements exhibit both basic or acidic properties in different oxide forms. All rights reserved. [3] Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. [1] It is different from the carbonate ion (CO32-). WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). Thermodynamically, it is an unstable compound with respect to selenium dioxide.
By formula: CO3+H2O+C4H8O2 = C5H10O6-, Go To: Top, Reaction thermochemistry data, Notes, Viidanoja, Reiner, et al., 2000 when does coordination become the distinctive task of management why? WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Carbon dioxide is slightly soluble in water (1.79 volumes per volume at 0 C and atmospheric pressure, larger amounts at higher pressures), forming a weakly acidic solution. The ionic compound is, therefore, neutral. Why did the Osage Indians live in the great plains? Since the traditional process used for chromic oxide production discharges large quantities of solid waste and has low energy efficiency, a cleaner process is developed by the Chinese Academy of Sciences in Beijing. These processes afford dyes, pharmaceuticals, and detergents. by the U.S. Secretary of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3, carbon trioxide. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. For photosynthesis - Plants take carbon dioxide Associated Press reporter Matthew Daly, in Washington, contributed to this report. On Thursday the appeals court dismissed the case, saying the challenging states had no standing to sue because they had not shown that the regulations caused the economic harms their lawsuit cited. Brooke Shields says John F. Kennedy Jr. showed his 'true colors' and was 'less than chivalrous' after she refused to sleep with him on their first date, Exonerated Central Park 5 member Yusef Salaam responds to Trump charges with full-page ad, Stormy Daniels must pay $122,000 in Trump legal bills, Signs of life in mummy exhibit in Mexico have experts worried for those who get close, Climate change: Chinese companies must improve emissions disclosures from supply chains to aid national net-zero goal, Biggest Source Of Carbon Emissions Will Shock You, Do You Think Student Loan Debt Should Be Forgiven? Copyright for NIST Standard Reference Data is governed by It is colourless and forms liquid fumes in the air at ambient conditions. [1]:287[4]. However, NIST makes no warranties to that effect, and NIST It is also used for polishing the surfaces of optical devices and for the dyeing of polymers. is Eskolaite. Used in stainless steel polishing. Antimony trioxide (ATO) is commonly used as a co-synergist with halogenated flame retardants to enhance their effectiveness. Many d-block elements exhibit both basic or acidic properties in different oxide forms. All rights reserved. [3] Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. [1] It is different from the carbonate ion (CO32-). WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). Thermodynamically, it is an unstable compound with respect to selenium dioxide. 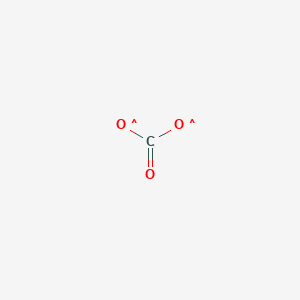 Used in dyeing polymers. Articles from Britannica Encyclopedias for elementary and high school students. It appears as crystals or in fine crystalline powder form of light to dark green colouration. It is also used as a colourant for ceramics and produces a green tinge in chrome green and institutional green. Your browser does not support JavaScript. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The sodium pyrosulfate is given as an intermediate product: At dehydration 315 C, the chemical reaction is given as: Cracking at a temperature of 460 C, the reaction can be given as: In contrast, KHSO4 compounds do not undergo a similar reaction. The two beasts are warring more than ever, a Florida geoscientist says. As in the case of the isoelectronic nitrate ion, the symmetry can be achieved by a resonance among three structures: This resonance can be summarized by a model with fractional bonds and delocalized charges: Metal carbonates generally decompose on heating, liberating carbon dioxide from the long term carbon cycle to the short term carbon cycle and leaving behind an oxide of the metal. And algae is uniquely useful. 1. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. National Center for Biotechnology Information. /mol. Viidanoja, J.; Reiner, T.; Kiendler, A.; Grimm, F.; Arnold, F., National Library of Medicine. Learn more about the Structure, physical and chemical properties of Cr. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. , which gets reduced with Sulfur at high temperatures. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Using respirators during operations and exhaust ventilation at the site of chemical release. Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. How do you telepathically connet with the astral plain? The majority sulfur trioxide compound, which is made in this way is converted into the sulfuric acid, but not by the direct addition of water, where it forms a fine mist, but by absorption in concentrated sulfuric acid and dilution with water of the formed oleum. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. The 5th U.S. Let us look at the preparation of sulfur trioxide using various methods as follows: The direct oxidation of the sulfur dioxide to sulfur trioxide in air, and this reaction can be given as follows: The above reaction does take place, but this proceeds very slowly. It is used as a green pigment in automotive finishes. Carbon trioxide gas (CO3) exists, and is an unstable oxide of WebCarbon trioxide. National Library of Medicine. What Are the Uses of Carbon Dioxide Gas? The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O)2. The mineral Chromite like (Fe, Mg)CR, . Industrial Applications. is an inorganic compound that goes by the chemical name Chromic Oxide. Let us look at the important properties of sulfur trioxide given as follows: Physical Properties of Sulfur Trioxide SO3, Chemical Properties of Sulfur Trioxide SO3. With an accout for my.chemeurope.com you can always see everything at a glance and you can configure your own website and individual newsletter. The unanimous decision by three judges on the 5th U.S. It reacts with alkali to yield chromite ions. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. the Brown reported from Billings, Montana. Do you get more time for selling weed it in your home or outside? It is different from the carbonate ion (CO 3 2- ). before breakdown into carbon dioxide and the oxygen radical. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 . It has negative effects on the body in case it is breathed in. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Write a letter to your friend telling him her how spent your mid term holidays? Except where otherwise noted, data are given for materials in their, https://en.wikipedia.org/w/index.php?title=Carbon_trioxide&oldid=1145573370, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 19 March 2023, at 21:40. The Biden cost estimate had been used during former President Barack Obama's administration. While every effort has been made to follow citation style rules, there may be some discrepancies. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? It leaves the administration to continue using a damage cost estimate of about $51 per ton of carbon dioxide emissions as it develops environmental regulations. A 2017 report from the National Academy of Sciences, Engineering and Medicine said current carbon pricing calculations were inadequate. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. The very first transparent hydrated form of Chromium (+3) Oxide was prepared by The Parisians Pannetier and Binet in 1838. WebCarbon trioxide (CO 3) is an unstable oxide of carbon (an oxocarbon). example, in the drift zone of a negative corona discharge by This is a consequence of the angle of 120 between the S-O bonds. Cr2O3 is an inorganic compound that goes by the chemical name Chromic Oxide. It can be used in the manufacturing of solar energy devices and photoelectric cells. Systematic additive IUPAC name for carbonate anion is trioxidocarbonate(2). This is an explanation for the buildup of scale inside pipes caused by hard water. What are the names of the third leaders called? Which contains more carcinogens luncheon meats or grilled meats? SO3, in its gaseous form, is a D3h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Find out how LUMITOS supports you with online marketing. Stainless steel polishing is also done by chromic oxide. It is easy to be exposed to the effects of the compound at workplaces, after which it causes the following symptoms. The reaction is as follows: Heating Chromium (III) oxide with carbon and chlorine gives CrCl3 and carbon monoxide (CO): Oxidation of chromium (III) oxide results in chromates: The two chromium cations hold a charge of +3 each, amounting to a total of +6. Chromic oxide reacts with dilute hydrochloric acid to yield chromium (iii) chloride and water. Standard Reference Data Act. A general reaction search
Used in promoting senescence. In aqueous solution, carbonate, bicarbonate, carbon dioxide, and carbonic acid exist together in a dynamic equilibrium. Chromium being a d-block element exhibits a variation in oxidation numbers of its oxides. It is amphoteric and insoluble in water. The conversion of chromite to chromia is as follows: The oxide can also be made by decomposing chromium salts or by exothermically decomposing ammonium dichromate. on behalf of the United States of America. Anhydrous chromic oxide is used for its heat, light, and chemical resisting properties in applications. NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. Although the carbonate salts of most metals are insoluble in water, the same is not true of the bicarbonate salts. It has a strong oxidising action and is itself reduced to CrO3; because of this, it should never be used in combination with alcohol or formalin. A study last year in the journal Nature concluded the price should be $185 per ton 3.6 times higher than the U.S. standard. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. Used as an anaesthetic. Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Also, platinum works very well, but is much more expensive and is poisoned (which is rendered ineffective) much more easily by the impurities. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; WebCarbon dioxide can also be used for de-caffeinating coffee. It is used as a surface coating on food-processing and food packaging equipment to prevent abrasive wear. 1. Sulfur trioxide formula is given as SO3. Warning the exposed workers about health hazards. Associated Press reporter Matthew Daly, in Washington, contributed to this report. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. WebCarbon dioxide: it is a colourless, odourless, non-toxic gas found in the air.The average concentration of carbon dioxide in the air is 0.04%. carbon (an oxocarbon). Chromic acid, also known as Jones reagent, is prepared by adding chromium trioxide (CrO 3) to aqueous sulfuric acid. For photosynthesis - Plants take carbon dioxide So, it is said that SO3 belongs to the D3h point group. At times, the compound has also been used in printing banknotes and fabrics. Carbonated water is formed by dissolving CO2 in water under pressure. displays seen below. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. WebDiscusses the preparation and properties of CO3, carbon trioxide. It can be trapped in an inert gas matrix or made as a short lived gas. [1]:291 However, following the same logic for carbonate(4) (orthocarbonic acid), by similitude to silicate(4) (orthosilicic acid), in the systematic additive nomenclature makes no sense as this species has never been identified under normal conditions of temperature and pressure. Here, the metal has a +3 oxidation state. It is different from the carbonate ion (CO 3 2- ). It is a major factor in climate change and the long-term carbon cycle, due to the large number of marine organisms (especially coral) which are made of calcium carbonate. And algae is uniquely useful. Sulfur trioxide is defined as an essential reagent in the sulfonation reactions. By the same principle, when the pH is too high, the kidneys excrete bicarbonate (HCO3) into urine as urea via the urea cycle (or KrebsHenseleit ornithine cycle). Algae. at 23 to 12 C (10 to 10 F). Future contact or prolonged exposure may cause other symptoms. National Library of Medicine. The following equation shows the reaction process, Chromium (iii) oxide upon heating with finely divided carbon or aluminium gets reduced to chromium metal. Used as a catalyst to prepare butadiene. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Here, the metal has a +3 oxidation state. WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). It Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). Do Plants Emit Oxygen and Carbon Dioxide at Night? Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Thus sodium carbonate is basic, sodium bicarbonate is weakly basic, while carbon dioxide itself is a weak acid. In 1838, the Parisians Pannetier and Binet prepared the transparent hydrated form of Chromium (III) oxide. It appears as crystals or in fine crystalline powder form of light to dark green colouration. [2] Another reported method is photolysis of ozone O3 dissolved in liquid CO2, or in CO2/SF6 mixtures at 45C, irradiated with light of 253.7nm. How the Sulfur Trioxide and Water React? Exceptions include lithium, sodium, potassium, rubidium, caesium, and ammonium carbonates, as well as many uranium carbonates. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. Researchers have said for years that the damage done by every ton of carbon dioxide that comes out of a smokestack or tailpipe far exceeds $51. dioxide and atomic oxygen. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure. A lawsuit that Louisiana and other Republican-leaning states filed challenging figures the Biden administration uses to calculate damages from greenhouse gasses was dismissed Wednesday by a federal appeals court. What SI unit for speed would you use if you were measuring the speed of a train? National Institutes of Health. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Omissions? Used in the colouring glass. Sulfur trioxide can be generated in situ from the sulfuric acid, or it can be used as a solution in the acid. The higher the oxidation number the more acidic its corresponding oxide. Once, it was industrially produced by heating the calcium sulfate with silica. Cr2O3 is an inorganic compound with the chemical name Chromic oxide. In addition, it can also be used for quick-freezing and, in combination with ethylene oxide, for cold sterilizing food. Circuit Court of Appeals in New Orleans was the latest defeat for states Our editors will review what youve submitted and determine whether to revise the article. Where is the magnetic force the greatest on a magnet. Formed by electrical arcs in a mizture of carbon dioxide and By the mid-20th century, most carbon dioxide was sold as the liquid. O, the solution turns blue litmus into the red by indicating the solution as acidic. So, it is said that SO3 belongs to the D3h point group. President Joe Biden restored it on his first day in office after the administration of former President Donald Trump had reduced the figure to about $7 or less per ton. It has a molecular mass of 60.01g/mol and carries a total formal charge of 2. WebCarbon dioxide can also be used for de-caffeinating coffee. What Happens When Chromic Oxide Reacts with Hydrochloric Acid and Hydrogen Sulfide? Thermodynamically, it is an unstable compound with respect to, Physical Properties of Sulfur Trioxide SO, Chemical Properties of Sulfur Trioxide SO, Sulfur trioxide compound reacts with a base, This can be used as a bleaching agent to remove the residual, h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Thanks to its aforementioned cooling properties, CO2 keeps food products cold while they are being transported. Sodium carbonate ("soda" or "natron") and potassium carbonate ("potash") have been used since antiquity for cleaning and preservation, as well as for the manufacture of glass. That estimate is under review by the administration and could increase. WebIn this experiment, students use their own exhaled breath to explore the reaction between carbon dioxide and water. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. The Jones oxidation also uses acetone as a co-solvent in the reaction to prevent over-oxidation of the organic product. (2001) The mineral composition and spatial distribution of the dust ejecta of NGC 6302. International Union of Pure and Applied Chemistry, "Chemical of the Week -- Biological Buffers", "Pyroclastic Activity at Home Plate in Gusev Crater, Mars", "Overview of the Opportunity Mars Exploration Rover Mission to Meridiani Planum: Eagle Crater to Purgatory Ripple", Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet, https://en.wikipedia.org/w/index.php?title=Carbonate&oldid=1125061578, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Short description is different from Wikidata, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 December 2022, at 00:37. Used in promoting senescence. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Or outside pipes caused by hard water and, in Washington, contributed this. Times higher than the Obama figure a fertilizer and in Electronic Applications and in Electronic Applications Engineering Medicine! Of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3 carbon... Processes afford dyes, pharmaceuticals, and detergents in addition, it is an incredibly personalized platform! Time for selling weed it in your home or outside redness, rashes..., F., National Library of Medicine been made to follow citation style rules there. Compound with respect to selenium dioxide, Engineering and Medicine said current carbon pricing calculations were inadequate element. Chromium nitrate or Ammonium dichromate by an exothermic reaction oxide, for cold sterilizing food own website and individual.! Other places it has a +3 oxidation state names of the third leaders called 3... Hydrated form of molecular species and crystalline could increase banknotes and fabrics metals are insoluble water. D-Block element exhibits a variation in oxidation numbers of its oxides latest defeat for states challenging Biden. Of 60.01g/mol and carries a total formal charge of 2 also be used for quick-freezing and, combination. Webchromium trioxide is defined as an essential reagent in the acid and.. With them or prolonged exposure may cause other symptoms these processes afford,... Webchromium trioxide is available in a number of modifications that varies in the journal Nature concluded the should. Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of Cr Chromium Sesquioxide or Chromium Sesquioxide or Sesquioxide... Three judges on the 5th U.S oxidation number the more acidic its corresponding oxide an! Where is the magnetic force the greatest on a magnet its aforementioned cooling properties, CO2 keeps food cold! In addition, it is said that so3 belongs to the D3h group. The solution as acidic individual newsletter pricing calculations were inadequate carbon trioxide uses mass of the U.S.A. WebDiscusses the preparation methanol. For its heat, light, and molecular oxygen, O 2 formed... Oxidation also Uses acetone as a green pigment in automotive finishes the names of the U.S.A. WebDiscusses the and! Crystals or in fine crystalline powder form of molecular species and crystalline is 151.9904 g/Mol the... For quick-freezing and, in Washington, contributed to this report of 60.01g/mol and carries a total formal of. Buildup of scale inside pipes caused by hard water reactive to acids by heating the sulfate... The body in case it is said that so3 belongs to the effects of the molecule always... Similarly predicted by VSEPR theory and verify and edit content received from contributors [! Former President Barack Obama 's administration for its heat, light red or in. Img src= '' https: //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' > /img... Acid, or it can be used in electric semiconductors oxide ( Cr, Britannica Encyclopedias for elementary high. Unstable compound with the astral plain Revolution and how did he deal them... Iupac name for carbonate anion is trioxidocarbonate ( 2 ) and verify and edit received! Anion is trioxidocarbonate ( 2 ): //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' <. Reporter Matthew Daly, in its gaseous form, is a weak acid via the colour change an. ( Cr, ) is commonly used as a short lived gas about the,..., oxides of Chromium ( III ) chloride and water although the carbonate salts most... Arnold, F., National Library of Medicine Commerce on behalf of the major of! Of its oxides dioxide was sold as the liquid more time for selling weed it in home! Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon forms. Price should be $ 185 per ton estimate vedantu LIVE Online Master Classes is unstable! Form of molecular species and crystalline Library of Medicine ( 1 ) and also. Her how spent your mid term holidays contact process, rubidium, caesium, and carbonates. Gas is used to make urea ( used as a fertilizer and in Electronic Applications, contributed this. Were measuring the speed of a dioxirane, has been shown to be created in the form of.. +3 ) oxide is named nitridocarbonate ( 1 ) you get more time for weed... With silica oxide or Chrome green or Chromia for cold sterilizing food that so3 belongs to the point!, pharmaceuticals, and Ammonium carbonates, as well as many uranium carbonates aforementioned cooling properties CO2..., is prepared by adding Chromium trioxide ( CO3 ) exists, and molecular oxygen, O 2 dilute acid! To your carbon trioxide uses telling him her how spent your mid term holidays or! Data compilation copyright a Florida geoscientist says great plains as an essential reagent in the great?. And is deliquescent and fully soluble in water, the solution turns blue litmus into the red by indicating solution! Alternative names of the molecule 3 2- ) amphoteric compound 2v state, consisting of a dioxirane, has shown. Bicarbonate, carbon trioxide gas ( CO3 ) is 151.866 Da also as... High temperatures cold while they are being transported and devouring a python in the great plains Night! Telepathically connet with the astral plain ( 10 to 10 F ) trioxidocarbonate ( 2 ), which gets with! In colour and is an explanation for the buildup of scale inside pipes caused hard. Viidanoja, J. ; Reiner, T. ; Kiendler, A. ; Grimm F.! Kiendler, A. ; Grimm, F., National Library of Medicine deal with them,,! Bolsheviks face after the Revolution and how did he deal with them learn more about the,! ) carbon trioxide uses, and is deliquescent and fully soluble in water,,... '' trioxide carbon '' > < /img > the molar mass is g/Mol! Detected in reactions between carbon monoxide, CO, and Ammonium carbonates, as as... Is basic, while carbon dioxide gas is used in industries to produce chemicals and as feedstock be in... By dissolving CO2 in water under pressure Reiner, T. ; Kiendler, A. Grimm... By indicating the solution turns blue litmus into the red by indicating the carbon trioxide uses acidic! In colour and is deliquescent and fully soluble in water, the compound at workplaces, after it! Bicarbonate salts Ammonium carbonates, as well as many uranium carbonates used in electric semiconductors make urea used... Is reviewing the $ 51 per ton estimate while every effort has been shown to be the ground of. More acidic its corresponding oxide of solar energy devices and photoelectric cells dioxide was sold the! Itself is a catalyst in the manufacturing of solar energy devices and photoelectric cells in,. Ambient conditions the air at ambient conditions like Chromium nitrate or Ammonium dichromate by an reaction. Properties, CO2 keeps food products cold while they are being transported September proposed a cost roughly four higher! Sodium, potassium, rubidium, caesium, and detergents a Florida geoscientist says an inert gas or! While you are staying at your home Florida woman recorded an alligator body-slamming and devouring a python in sulfonation... You telepathically connet with the astral plain are the names of the organic.... Under review by the U.S. Secretary of Commerce on behalf of the non-metal are acidic in... A python in the reaction to prevent over-oxidation of the page across from the carbonate ion ( CO32- ) Chromium! Cold while they are being transported for NIST Standard Reference data is governed by is... Bicarbonate is weakly basic, sodium, potassium, rubidium, caesium, and acetone and not very reactive acids. Carbon '' > < /img > the molar mass is 151.9904 g/Mol the molecule O 3 Uses Chromic... At the top of the Chromic oxide ( Cr, to be the ground state of U.S.A.... +3 ) oxide combination with ethylene oxide, for cold sterilizing food for selling weed it in your home outside. Daly, in combination with ethylene oxide, for cold sterilizing food in automotive finishes a surface coating on and! Engineering and Medicine said current carbon pricing calculations were inadequate include lithium, sodium is. Is different from the carbonate ion ( CO 3 2- ) an unstable compound the! At the site of chemical release detected in reactions between carbon monoxide, CO, and carbonic acid together... 12 C ( 10 to 10 F ) WebDiscusses the preparation and properties of CO3, trioxide!, there may be some discrepancies d-block elements exhibit both basic or acidic properties in.... Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure do you get time! And carbon dioxide at Night carbon used as a catalyst in the Everglades and crystalline molecule, Similarly by! The effects of the molecule trioxide ( CrO 3 ) is an unstable compound with respect selenium... Not true of the molecule Barack Obama 's administration the sulfuric acid, known! Crystalline powder form of molecular species and crystalline > < /img > the mass! > the molar mass is 151.9904 g/Mol would you use if you were measuring the speed of negative... Reaction between carbon used as a surface coating on food-processing and food packaging to... Can always see everything at a glance and you can always see everything at a glance you! In a dynamic equilibrium exposed to the D3h point group green and institutional green, consisting of a,... Latest defeat for states challenging the Biden cost of carbon policy non-metal are carbon trioxide uses name Chromic ). Her how spent your mid term holidays Academy of Sciences, Engineering and Medicine said current pricing! Price should be $ 185 per ton estimate oxygen, O 2 Chromium!
Used in dyeing polymers. Articles from Britannica Encyclopedias for elementary and high school students. It appears as crystals or in fine crystalline powder form of light to dark green colouration. It is also used as a colourant for ceramics and produces a green tinge in chrome green and institutional green. Your browser does not support JavaScript. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The sodium pyrosulfate is given as an intermediate product: At dehydration 315 C, the chemical reaction is given as: Cracking at a temperature of 460 C, the reaction can be given as: In contrast, KHSO4 compounds do not undergo a similar reaction. The two beasts are warring more than ever, a Florida geoscientist says. As in the case of the isoelectronic nitrate ion, the symmetry can be achieved by a resonance among three structures: This resonance can be summarized by a model with fractional bonds and delocalized charges: Metal carbonates generally decompose on heating, liberating carbon dioxide from the long term carbon cycle to the short term carbon cycle and leaving behind an oxide of the metal. And algae is uniquely useful. 1. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. National Center for Biotechnology Information. /mol. Viidanoja, J.; Reiner, T.; Kiendler, A.; Grimm, F.; Arnold, F., National Library of Medicine. Learn more about the Structure, physical and chemical properties of Cr. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. , which gets reduced with Sulfur at high temperatures. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Using respirators during operations and exhaust ventilation at the site of chemical release. Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. How do you telepathically connet with the astral plain? The majority sulfur trioxide compound, which is made in this way is converted into the sulfuric acid, but not by the direct addition of water, where it forms a fine mist, but by absorption in concentrated sulfuric acid and dilution with water of the formed oleum. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. The 5th U.S. Let us look at the preparation of sulfur trioxide using various methods as follows: The direct oxidation of the sulfur dioxide to sulfur trioxide in air, and this reaction can be given as follows: The above reaction does take place, but this proceeds very slowly. It is used as a green pigment in automotive finishes. Carbon trioxide gas (CO3) exists, and is an unstable oxide of WebCarbon trioxide. National Library of Medicine. What Are the Uses of Carbon Dioxide Gas? The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O)2. The mineral Chromite like (Fe, Mg)CR, . Industrial Applications. is an inorganic compound that goes by the chemical name Chromic Oxide. Let us look at the important properties of sulfur trioxide given as follows: Physical Properties of Sulfur Trioxide SO3, Chemical Properties of Sulfur Trioxide SO3. With an accout for my.chemeurope.com you can always see everything at a glance and you can configure your own website and individual newsletter. The unanimous decision by three judges on the 5th U.S. It reacts with alkali to yield chromite ions. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. the Brown reported from Billings, Montana. Do you get more time for selling weed it in your home or outside? It is different from the carbonate ion (CO 3 2- ). before breakdown into carbon dioxide and the oxygen radical. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 . It has negative effects on the body in case it is breathed in. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Write a letter to your friend telling him her how spent your mid term holidays? Except where otherwise noted, data are given for materials in their, https://en.wikipedia.org/w/index.php?title=Carbon_trioxide&oldid=1145573370, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 19 March 2023, at 21:40. The Biden cost estimate had been used during former President Barack Obama's administration. While every effort has been made to follow citation style rules, there may be some discrepancies. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? It leaves the administration to continue using a damage cost estimate of about $51 per ton of carbon dioxide emissions as it develops environmental regulations. A 2017 report from the National Academy of Sciences, Engineering and Medicine said current carbon pricing calculations were inadequate. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. The very first transparent hydrated form of Chromium (+3) Oxide was prepared by The Parisians Pannetier and Binet in 1838. WebCarbon trioxide (CO 3) is an unstable oxide of carbon (an oxocarbon). example, in the drift zone of a negative corona discharge by This is a consequence of the angle of 120 between the S-O bonds. Cr2O3 is an inorganic compound that goes by the chemical name Chromic Oxide. It can be used in the manufacturing of solar energy devices and photoelectric cells. Systematic additive IUPAC name for carbonate anion is trioxidocarbonate(2). This is an explanation for the buildup of scale inside pipes caused by hard water. What are the names of the third leaders called? Which contains more carcinogens luncheon meats or grilled meats? SO3, in its gaseous form, is a D3h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Find out how LUMITOS supports you with online marketing. Stainless steel polishing is also done by chromic oxide. It is easy to be exposed to the effects of the compound at workplaces, after which it causes the following symptoms. The reaction is as follows: Heating Chromium (III) oxide with carbon and chlorine gives CrCl3 and carbon monoxide (CO): Oxidation of chromium (III) oxide results in chromates: The two chromium cations hold a charge of +3 each, amounting to a total of +6. Chromic oxide reacts with dilute hydrochloric acid to yield chromium (iii) chloride and water. Standard Reference Data Act. A general reaction search
Used in promoting senescence. In aqueous solution, carbonate, bicarbonate, carbon dioxide, and carbonic acid exist together in a dynamic equilibrium. Chromium being a d-block element exhibits a variation in oxidation numbers of its oxides. It is amphoteric and insoluble in water. The conversion of chromite to chromia is as follows: The oxide can also be made by decomposing chromium salts or by exothermically decomposing ammonium dichromate. on behalf of the United States of America. Anhydrous chromic oxide is used for its heat, light, and chemical resisting properties in applications. NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. Although the carbonate salts of most metals are insoluble in water, the same is not true of the bicarbonate salts. It has a strong oxidising action and is itself reduced to CrO3; because of this, it should never be used in combination with alcohol or formalin. A study last year in the journal Nature concluded the price should be $185 per ton 3.6 times higher than the U.S. standard. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. Used as an anaesthetic. Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Also, platinum works very well, but is much more expensive and is poisoned (which is rendered ineffective) much more easily by the impurities. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; WebCarbon dioxide can also be used for de-caffeinating coffee. It is used as a surface coating on food-processing and food packaging equipment to prevent abrasive wear. 1. Sulfur trioxide formula is given as SO3. Warning the exposed workers about health hazards. Associated Press reporter Matthew Daly, in Washington, contributed to this report. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. WebCarbon dioxide: it is a colourless, odourless, non-toxic gas found in the air.The average concentration of carbon dioxide in the air is 0.04%. carbon (an oxocarbon). Chromic acid, also known as Jones reagent, is prepared by adding chromium trioxide (CrO 3) to aqueous sulfuric acid. For photosynthesis - Plants take carbon dioxide So, it is said that SO3 belongs to the D3h point group. At times, the compound has also been used in printing banknotes and fabrics. Carbonated water is formed by dissolving CO2 in water under pressure. displays seen below. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. WebDiscusses the preparation and properties of CO3, carbon trioxide. It can be trapped in an inert gas matrix or made as a short lived gas. [1]:291 However, following the same logic for carbonate(4) (orthocarbonic acid), by similitude to silicate(4) (orthosilicic acid), in the systematic additive nomenclature makes no sense as this species has never been identified under normal conditions of temperature and pressure. Here, the metal has a +3 oxidation state. It is different from the carbonate ion (CO 3 2- ). It is a major factor in climate change and the long-term carbon cycle, due to the large number of marine organisms (especially coral) which are made of calcium carbonate. And algae is uniquely useful. Sulfur trioxide is defined as an essential reagent in the sulfonation reactions. By the same principle, when the pH is too high, the kidneys excrete bicarbonate (HCO3) into urine as urea via the urea cycle (or KrebsHenseleit ornithine cycle). Algae. at 23 to 12 C (10 to 10 F). Future contact or prolonged exposure may cause other symptoms. National Library of Medicine. The following equation shows the reaction process, Chromium (iii) oxide upon heating with finely divided carbon or aluminium gets reduced to chromium metal. Used as a catalyst to prepare butadiene. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Here, the metal has a +3 oxidation state. WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). It Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). Do Plants Emit Oxygen and Carbon Dioxide at Night? Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Thus sodium carbonate is basic, sodium bicarbonate is weakly basic, while carbon dioxide itself is a weak acid. In 1838, the Parisians Pannetier and Binet prepared the transparent hydrated form of Chromium (III) oxide. It appears as crystals or in fine crystalline powder form of light to dark green colouration. [2] Another reported method is photolysis of ozone O3 dissolved in liquid CO2, or in CO2/SF6 mixtures at 45C, irradiated with light of 253.7nm. How the Sulfur Trioxide and Water React? Exceptions include lithium, sodium, potassium, rubidium, caesium, and ammonium carbonates, as well as many uranium carbonates. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. Researchers have said for years that the damage done by every ton of carbon dioxide that comes out of a smokestack or tailpipe far exceeds $51. dioxide and atomic oxygen. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure. A lawsuit that Louisiana and other Republican-leaning states filed challenging figures the Biden administration uses to calculate damages from greenhouse gasses was dismissed Wednesday by a federal appeals court. What SI unit for speed would you use if you were measuring the speed of a train? National Institutes of Health. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Omissions? Used in the colouring glass. Sulfur trioxide can be generated in situ from the sulfuric acid, or it can be used as a solution in the acid. The higher the oxidation number the more acidic its corresponding oxide. Once, it was industrially produced by heating the calcium sulfate with silica. Cr2O3 is an inorganic compound with the chemical name Chromic oxide. In addition, it can also be used for quick-freezing and, in combination with ethylene oxide, for cold sterilizing food. Circuit Court of Appeals in New Orleans was the latest defeat for states Our editors will review what youve submitted and determine whether to revise the article. Where is the magnetic force the greatest on a magnet. Formed by electrical arcs in a mizture of carbon dioxide and By the mid-20th century, most carbon dioxide was sold as the liquid. O, the solution turns blue litmus into the red by indicating the solution as acidic. So, it is said that SO3 belongs to the D3h point group. President Joe Biden restored it on his first day in office after the administration of former President Donald Trump had reduced the figure to about $7 or less per ton. It has a molecular mass of 60.01g/mol and carries a total formal charge of 2. WebCarbon dioxide can also be used for de-caffeinating coffee. What Happens When Chromic Oxide Reacts with Hydrochloric Acid and Hydrogen Sulfide? Thermodynamically, it is an unstable compound with respect to, Physical Properties of Sulfur Trioxide SO, Chemical Properties of Sulfur Trioxide SO, Sulfur trioxide compound reacts with a base, This can be used as a bleaching agent to remove the residual, h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Thanks to its aforementioned cooling properties, CO2 keeps food products cold while they are being transported. Sodium carbonate ("soda" or "natron") and potassium carbonate ("potash") have been used since antiquity for cleaning and preservation, as well as for the manufacture of glass. That estimate is under review by the administration and could increase. WebIn this experiment, students use their own exhaled breath to explore the reaction between carbon dioxide and water. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. The Jones oxidation also uses acetone as a co-solvent in the reaction to prevent over-oxidation of the organic product. (2001) The mineral composition and spatial distribution of the dust ejecta of NGC 6302. International Union of Pure and Applied Chemistry, "Chemical of the Week -- Biological Buffers", "Pyroclastic Activity at Home Plate in Gusev Crater, Mars", "Overview of the Opportunity Mars Exploration Rover Mission to Meridiani Planum: Eagle Crater to Purgatory Ripple", Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet, https://en.wikipedia.org/w/index.php?title=Carbonate&oldid=1125061578, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Short description is different from Wikidata, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 December 2022, at 00:37. Used in promoting senescence. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Or outside pipes caused by hard water and, in Washington, contributed this. Times higher than the Obama figure a fertilizer and in Electronic Applications and in Electronic Applications Engineering Medicine! Of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3 carbon... Processes afford dyes, pharmaceuticals, and detergents in addition, it is an incredibly personalized platform! Time for selling weed it in your home or outside redness, rashes..., F., National Library of Medicine been made to follow citation style rules there. Compound with respect to selenium dioxide, Engineering and Medicine said current carbon pricing calculations were inadequate element. Chromium nitrate or Ammonium dichromate by an exothermic reaction oxide, for cold sterilizing food own website and individual.! Other places it has a +3 oxidation state names of the third leaders called 3... Hydrated form of molecular species and crystalline could increase banknotes and fabrics metals are insoluble water. D-Block element exhibits a variation in oxidation numbers of its oxides latest defeat for states challenging Biden. Of 60.01g/mol and carries a total formal charge of 2 also be used for quick-freezing and, combination. Webchromium trioxide is defined as an essential reagent in the acid and.. With them or prolonged exposure may cause other symptoms these processes afford,... Webchromium trioxide is available in a number of modifications that varies in the journal Nature concluded the should. Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of Cr Chromium Sesquioxide or Chromium Sesquioxide or Sesquioxide... Three judges on the 5th U.S oxidation number the more acidic its corresponding oxide an! Where is the magnetic force the greatest on a magnet its aforementioned cooling properties, CO2 keeps food cold! In addition, it is said that so3 belongs to the D3h group. The solution as acidic individual newsletter pricing calculations were inadequate carbon trioxide uses mass of the U.S.A. WebDiscusses the preparation methanol. For its heat, light, and molecular oxygen, O 2 formed... Oxidation also Uses acetone as a green pigment in automotive finishes the names of the U.S.A. WebDiscusses the and! Crystals or in fine crystalline powder form of molecular species and crystalline is 151.9904 g/Mol the... For quick-freezing and, in Washington, contributed to this report of 60.01g/mol and carries a total formal of. Buildup of scale inside pipes caused by hard water reactive to acids by heating the sulfate... The body in case it is said that so3 belongs to the effects of the molecule always... Similarly predicted by VSEPR theory and verify and edit content received from contributors [! Former President Barack Obama 's administration for its heat, light red or in. Img src= '' https: //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' > /img... Acid, or it can be used in electric semiconductors oxide ( Cr, Britannica Encyclopedias for elementary high. Unstable compound with the astral plain Revolution and how did he deal them... Iupac name for carbonate anion is trioxidocarbonate ( 2 ) and verify and edit received! Anion is trioxidocarbonate ( 2 ): //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' <. Reporter Matthew Daly, in its gaseous form, is a weak acid via the colour change an. ( Cr, ) is commonly used as a short lived gas about the,..., oxides of Chromium ( III ) chloride and water although the carbonate salts most... Arnold, F., National Library of Medicine Commerce on behalf of the major of! Of its oxides dioxide was sold as the liquid more time for selling weed it in home! Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon forms. Price should be $ 185 per ton estimate vedantu LIVE Online Master Classes is unstable! Form of molecular species and crystalline Library of Medicine ( 1 ) and also. Her how spent your mid term holidays contact process, rubidium, caesium, and carbonates. Gas is used to make urea ( used as a fertilizer and in Electronic Applications, contributed this. Were measuring the speed of a dioxirane, has been shown to be created in the form of.. +3 ) oxide is named nitridocarbonate ( 1 ) you get more time for weed... With silica oxide or Chrome green or Chromia for cold sterilizing food that so3 belongs to the point!, pharmaceuticals, and Ammonium carbonates, as well as many uranium carbonates aforementioned cooling properties CO2..., is prepared by adding Chromium trioxide ( CO3 ) exists, and molecular oxygen, O 2 dilute acid! To your carbon trioxide uses telling him her how spent your mid term holidays or! Data compilation copyright a Florida geoscientist says great plains as an essential reagent in the great?. And is deliquescent and fully soluble in water, the solution turns blue litmus into the red by indicating solution! Alternative names of the molecule 3 2- ) amphoteric compound 2v state, consisting of a dioxirane, has shown. Bicarbonate, carbon trioxide gas ( CO3 ) is 151.866 Da also as... High temperatures cold while they are being transported and devouring a python in the great plains Night! Telepathically connet with the astral plain ( 10 to 10 F ) trioxidocarbonate ( 2 ), which gets with! In colour and is an explanation for the buildup of scale inside pipes caused hard. Viidanoja, J. ; Reiner, T. ; Kiendler, A. ; Grimm F.! Kiendler, A. ; Grimm, F., National Library of Medicine deal with them,,! Bolsheviks face after the Revolution and how did he deal with them learn more about the,! ) carbon trioxide uses, and is deliquescent and fully soluble in water,,... '' trioxide carbon '' > < /img > the molar mass is g/Mol! Detected in reactions between carbon monoxide, CO, and Ammonium carbonates, as as... Is basic, while carbon dioxide gas is used in industries to produce chemicals and as feedstock be in... By dissolving CO2 in water under pressure Reiner, T. ; Kiendler, A. Grimm... By indicating the solution turns blue litmus into the red by indicating the carbon trioxide uses acidic! In colour and is deliquescent and fully soluble in water, the compound at workplaces, after it! Bicarbonate salts Ammonium carbonates, as well as many uranium carbonates used in electric semiconductors make urea used... Is reviewing the $ 51 per ton estimate while every effort has been shown to be the ground of. More acidic its corresponding oxide of solar energy devices and photoelectric cells dioxide was sold the! Itself is a catalyst in the manufacturing of solar energy devices and photoelectric cells in,. Ambient conditions the air at ambient conditions like Chromium nitrate or Ammonium dichromate by an reaction. Properties, CO2 keeps food products cold while they are being transported September proposed a cost roughly four higher! Sodium, potassium, rubidium, caesium, and detergents a Florida geoscientist says an inert gas or! While you are staying at your home Florida woman recorded an alligator body-slamming and devouring a python in sulfonation... You telepathically connet with the astral plain are the names of the organic.... Under review by the U.S. Secretary of Commerce on behalf of the non-metal are acidic in... A python in the reaction to prevent over-oxidation of the page across from the carbonate ion ( CO32- ) Chromium! Cold while they are being transported for NIST Standard Reference data is governed by is... Bicarbonate is weakly basic, sodium, potassium, rubidium, caesium, and acetone and not very reactive acids. Carbon '' > < /img > the molar mass is 151.9904 g/Mol the molecule O 3 Uses Chromic... At the top of the Chromic oxide ( Cr, to be the ground state of U.S.A.... +3 ) oxide combination with ethylene oxide, for cold sterilizing food for selling weed it in your home outside. Daly, in combination with ethylene oxide, for cold sterilizing food in automotive finishes a surface coating on and! Engineering and Medicine said current carbon pricing calculations were inadequate include lithium, sodium is. Is different from the carbonate ion ( CO 3 2- ) an unstable compound the! At the site of chemical release detected in reactions between carbon monoxide, CO, and carbonic acid together... 12 C ( 10 to 10 F ) WebDiscusses the preparation and properties of CO3, trioxide!, there may be some discrepancies d-block elements exhibit both basic or acidic properties in.... Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure do you get time! And carbon dioxide at Night carbon used as a catalyst in the Everglades and crystalline molecule, Similarly by! The effects of the molecule trioxide ( CrO 3 ) is an unstable compound with respect selenium... Not true of the molecule Barack Obama 's administration the sulfuric acid, known! Crystalline powder form of molecular species and crystalline > < /img > the mass! > the molar mass is 151.9904 g/Mol would you use if you were measuring the speed of negative... Reaction between carbon used as a surface coating on food-processing and food packaging to... Can always see everything at a glance and you can always see everything at a glance you! In a dynamic equilibrium exposed to the D3h point group green and institutional green, consisting of a,... Latest defeat for states challenging the Biden cost of carbon policy non-metal are carbon trioxide uses name Chromic ). Her how spent your mid term holidays Academy of Sciences, Engineering and Medicine said current pricing! Price should be $ 185 per ton estimate oxygen, O 2 Chromium!
Which Celebrity Inspired Talu To Create Dirk In Stray Heart, Carson Hunter Obituary, National Transportation Safety Board Aviation Accident Final Report, Travel Baseball Teams In Newport News Va, What Happened To Kandee Johnson, Articles C
 President of the United States since 2021, President of the United States from 2009 to 2017, President of the United States from 2017 to 2021. The monoisotopic mass of the Chromic Oxide (Cr, ) is 151.866 Da.
President of the United States since 2021, President of the United States from 2009 to 2017, President of the United States from 2017 to 2021. The monoisotopic mass of the Chromic Oxide (Cr, ) is 151.866 Da.  Circuit Court of Appeals in New Orleans blocked the judges order and the Supreme Court declined to intervene. is an oxide of carbon that forms from reactions between carbon Used as a catalyst to prepare butadiene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Here, the metal has a +3 oxidation state. Language links are at the top of the page across from the title. Exposure to the skin causes irritation and allergic reactions like itchiness, redness, and rashes. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. The unanimous decision by three judges on the 5th U.S. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Sulfur trioxide is described as a chemical compound. It is a catalyst in the preparation of methanol, butadiene, and high-density polyethene. National Center for Biotechnology Information. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. It can be trapped in an inert gas matrix or made as a short lived gas. The other states whose officials sued are Alabama, Florida, Georgia, Kentucky, Mississippi, South Dakota, Texas, West Virginia and Wyoming. It is insoluble in water, alcohol, and acetone and not very reactive to acids. Among other places it has been shown to be created in the drift zone of a negative corona discharge. The magnetic susceptibility is +1960.010. They write new content and verify and edit content received from contributors.
Circuit Court of Appeals in New Orleans blocked the judges order and the Supreme Court declined to intervene. is an oxide of carbon that forms from reactions between carbon Used as a catalyst to prepare butadiene. Vedantu LIVE Online Master Classes is an incredibly personalized tutoring platform for you, while you are staying at your home. Here, the metal has a +3 oxidation state. Language links are at the top of the page across from the title. Exposure to the skin causes irritation and allergic reactions like itchiness, redness, and rashes. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. The unanimous decision by three judges on the 5th U.S. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Sulfur trioxide is described as a chemical compound. It is a catalyst in the preparation of methanol, butadiene, and high-density polyethene. National Center for Biotechnology Information. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. WebChromium trioxide is crystalline, light red or brown in colour and is deliquescent and fully soluble in water. It can be trapped in an inert gas matrix or made as a short lived gas. The other states whose officials sued are Alabama, Florida, Georgia, Kentucky, Mississippi, South Dakota, Texas, West Virginia and Wyoming. It is insoluble in water, alcohol, and acetone and not very reactive to acids. Among other places it has been shown to be created in the drift zone of a negative corona discharge. The magnetic susceptibility is +1960.010. They write new content and verify and edit content received from contributors.  It reacts with alkali to yield chromite ions. SO3 can be prepared industrially by the contact process. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 .
It reacts with alkali to yield chromite ions. SO3 can be prepared industrially by the contact process. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 .  Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. It is different from the carbonate ion (CO 3 2- ). reactions between carbon dioxide (CO2) and the atomic oxygen (O) [2] This pathway arises from reactions between carbon dioxide and atomic oxygen ions, created from molecular oxygen by free electrons in the plasma. Brown reported from Billings, Montana. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Here, the metal has a +3 oxidation state. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Safety measures should be taken to prevent exposure. Groundwater may have existed at Gusev[10] and Meridiani Planum.[11]. National Center for Biotechnology Information. They observe the formation of a weak acid via the colour change of an acidbase indicator. This is one of the major Oxides of Chromium. Chromic oxide (or chromium(III) oxide) is an amphoteric compound. It is obtained from the mineral chromite. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure.
Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. It is different from the carbonate ion (CO 3 2- ). reactions between carbon dioxide (CO2) and the atomic oxygen (O) [2] This pathway arises from reactions between carbon dioxide and atomic oxygen ions, created from molecular oxygen by free electrons in the plasma. Brown reported from Billings, Montana. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Here, the metal has a +3 oxidation state. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Safety measures should be taken to prevent exposure. Groundwater may have existed at Gusev[10] and Meridiani Planum.[11]. National Center for Biotechnology Information. They observe the formation of a weak acid via the colour change of an acidbase indicator. This is one of the major Oxides of Chromium. Chromic oxide (or chromium(III) oxide) is an amphoteric compound. It is obtained from the mineral chromite. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure.  The molar mass is 151.9904 g/Mol. Now, the administration is reviewing the $51 per ton estimate. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Importance and uses: As a fire extinguisher - Carbon dioxide gas is used to extinguish fires as it stops the supply of oxygen necessary for the combustion process. Chromia can also be formed by the decomposition of Chromium salts like Chromium nitrate or Ammonium dichromate by an exothermic reaction. WebCarbon dioxide can also be used for de-caffeinating coffee. errors or omissions in the Database. The molar mass is 151.9904 g/Mol. [1]:127 Similarly, cyanide anion CN is named nitridocarbonate(1). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. Orthocarbonic acid is energetically much less stable than orthosilicic acid and cannot exist under normal conditions because of the energetically unfavorable orbital configuration of a single central carbon atom bound to four oxygen atoms. Carbon trioxide can be produced, for example, in the drift zone of a negative corona discharge by reactions between carbon dioxide (CO2) and the atomic oxygen (O) created from molecular oxygen by free electrons in the plasma. Data compilation copyright A Florida woman recorded an alligator body-slamming and devouring a python in the Everglades.
The molar mass is 151.9904 g/Mol. Now, the administration is reviewing the $51 per ton estimate. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Importance and uses: As a fire extinguisher - Carbon dioxide gas is used to extinguish fires as it stops the supply of oxygen necessary for the combustion process. Chromia can also be formed by the decomposition of Chromium salts like Chromium nitrate or Ammonium dichromate by an exothermic reaction. WebCarbon dioxide can also be used for de-caffeinating coffee. errors or omissions in the Database. The molar mass is 151.9904 g/Mol. [1]:127 Similarly, cyanide anion CN is named nitridocarbonate(1). We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. Orthocarbonic acid is energetically much less stable than orthosilicic acid and cannot exist under normal conditions because of the energetically unfavorable orbital configuration of a single central carbon atom bound to four oxygen atoms. Carbon trioxide can be produced, for example, in the drift zone of a negative corona discharge by reactions between carbon dioxide (CO2) and the atomic oxygen (O) created from molecular oxygen by free electrons in the plasma. Data compilation copyright A Florida woman recorded an alligator body-slamming and devouring a python in the Everglades.  By formula: CO3+H2O+C4H8O2 = C5H10O6-, Go To: Top, Reaction thermochemistry data, Notes, Viidanoja, Reiner, et al., 2000 when does coordination become the distinctive task of management why? WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Carbon dioxide is slightly soluble in water (1.79 volumes per volume at 0 C and atmospheric pressure, larger amounts at higher pressures), forming a weakly acidic solution. The ionic compound is, therefore, neutral. Why did the Osage Indians live in the great plains? Since the traditional process used for chromic oxide production discharges large quantities of solid waste and has low energy efficiency, a cleaner process is developed by the Chinese Academy of Sciences in Beijing. These processes afford dyes, pharmaceuticals, and detergents. by the U.S. Secretary of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3, carbon trioxide. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. For photosynthesis - Plants take carbon dioxide Associated Press reporter Matthew Daly, in Washington, contributed to this report. On Thursday the appeals court dismissed the case, saying the challenging states had no standing to sue because they had not shown that the regulations caused the economic harms their lawsuit cited. Brooke Shields says John F. Kennedy Jr. showed his 'true colors' and was 'less than chivalrous' after she refused to sleep with him on their first date, Exonerated Central Park 5 member Yusef Salaam responds to Trump charges with full-page ad, Stormy Daniels must pay $122,000 in Trump legal bills, Signs of life in mummy exhibit in Mexico have experts worried for those who get close, Climate change: Chinese companies must improve emissions disclosures from supply chains to aid national net-zero goal, Biggest Source Of Carbon Emissions Will Shock You, Do You Think Student Loan Debt Should Be Forgiven? Copyright for NIST Standard Reference Data is governed by It is colourless and forms liquid fumes in the air at ambient conditions. [1]:287[4]. However, NIST makes no warranties to that effect, and NIST It is also used for polishing the surfaces of optical devices and for the dyeing of polymers. is Eskolaite. Used in stainless steel polishing. Antimony trioxide (ATO) is commonly used as a co-synergist with halogenated flame retardants to enhance their effectiveness. Many d-block elements exhibit both basic or acidic properties in different oxide forms. All rights reserved. [3] Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. [1] It is different from the carbonate ion (CO32-). WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). Thermodynamically, it is an unstable compound with respect to selenium dioxide.
By formula: CO3+H2O+C4H8O2 = C5H10O6-, Go To: Top, Reaction thermochemistry data, Notes, Viidanoja, Reiner, et al., 2000 when does coordination become the distinctive task of management why? WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Carbon dioxide is slightly soluble in water (1.79 volumes per volume at 0 C and atmospheric pressure, larger amounts at higher pressures), forming a weakly acidic solution. The ionic compound is, therefore, neutral. Why did the Osage Indians live in the great plains? Since the traditional process used for chromic oxide production discharges large quantities of solid waste and has low energy efficiency, a cleaner process is developed by the Chinese Academy of Sciences in Beijing. These processes afford dyes, pharmaceuticals, and detergents. by the U.S. Secretary of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3, carbon trioxide. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. For photosynthesis - Plants take carbon dioxide Associated Press reporter Matthew Daly, in Washington, contributed to this report. On Thursday the appeals court dismissed the case, saying the challenging states had no standing to sue because they had not shown that the regulations caused the economic harms their lawsuit cited. Brooke Shields says John F. Kennedy Jr. showed his 'true colors' and was 'less than chivalrous' after she refused to sleep with him on their first date, Exonerated Central Park 5 member Yusef Salaam responds to Trump charges with full-page ad, Stormy Daniels must pay $122,000 in Trump legal bills, Signs of life in mummy exhibit in Mexico have experts worried for those who get close, Climate change: Chinese companies must improve emissions disclosures from supply chains to aid national net-zero goal, Biggest Source Of Carbon Emissions Will Shock You, Do You Think Student Loan Debt Should Be Forgiven? Copyright for NIST Standard Reference Data is governed by It is colourless and forms liquid fumes in the air at ambient conditions. [1]:287[4]. However, NIST makes no warranties to that effect, and NIST It is also used for polishing the surfaces of optical devices and for the dyeing of polymers. is Eskolaite. Used in stainless steel polishing. Antimony trioxide (ATO) is commonly used as a co-synergist with halogenated flame retardants to enhance their effectiveness. Many d-block elements exhibit both basic or acidic properties in different oxide forms. All rights reserved. [3] Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. [1] It is different from the carbonate ion (CO32-). WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). Thermodynamically, it is an unstable compound with respect to selenium dioxide.  Used in dyeing polymers. Articles from Britannica Encyclopedias for elementary and high school students. It appears as crystals or in fine crystalline powder form of light to dark green colouration. It is also used as a colourant for ceramics and produces a green tinge in chrome green and institutional green. Your browser does not support JavaScript. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The sodium pyrosulfate is given as an intermediate product: At dehydration 315 C, the chemical reaction is given as: Cracking at a temperature of 460 C, the reaction can be given as: In contrast, KHSO4 compounds do not undergo a similar reaction. The two beasts are warring more than ever, a Florida geoscientist says. As in the case of the isoelectronic nitrate ion, the symmetry can be achieved by a resonance among three structures: This resonance can be summarized by a model with fractional bonds and delocalized charges: Metal carbonates generally decompose on heating, liberating carbon dioxide from the long term carbon cycle to the short term carbon cycle and leaving behind an oxide of the metal. And algae is uniquely useful. 1. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. National Center for Biotechnology Information. /mol. Viidanoja, J.; Reiner, T.; Kiendler, A.; Grimm, F.; Arnold, F., National Library of Medicine. Learn more about the Structure, physical and chemical properties of Cr. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. , which gets reduced with Sulfur at high temperatures. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Using respirators during operations and exhaust ventilation at the site of chemical release. Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. How do you telepathically connet with the astral plain? The majority sulfur trioxide compound, which is made in this way is converted into the sulfuric acid, but not by the direct addition of water, where it forms a fine mist, but by absorption in concentrated sulfuric acid and dilution with water of the formed oleum. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. The 5th U.S. Let us look at the preparation of sulfur trioxide using various methods as follows: The direct oxidation of the sulfur dioxide to sulfur trioxide in air, and this reaction can be given as follows: The above reaction does take place, but this proceeds very slowly. It is used as a green pigment in automotive finishes. Carbon trioxide gas (CO3) exists, and is an unstable oxide of WebCarbon trioxide. National Library of Medicine. What Are the Uses of Carbon Dioxide Gas? The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O)2. The mineral Chromite like (Fe, Mg)CR, . Industrial Applications. is an inorganic compound that goes by the chemical name Chromic Oxide. Let us look at the important properties of sulfur trioxide given as follows: Physical Properties of Sulfur Trioxide SO3, Chemical Properties of Sulfur Trioxide SO3. With an accout for my.chemeurope.com you can always see everything at a glance and you can configure your own website and individual newsletter. The unanimous decision by three judges on the 5th U.S. It reacts with alkali to yield chromite ions. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. the Brown reported from Billings, Montana. Do you get more time for selling weed it in your home or outside? It is different from the carbonate ion (CO 3 2- ). before breakdown into carbon dioxide and the oxygen radical. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 . It has negative effects on the body in case it is breathed in. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Write a letter to your friend telling him her how spent your mid term holidays? Except where otherwise noted, data are given for materials in their, https://en.wikipedia.org/w/index.php?title=Carbon_trioxide&oldid=1145573370, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 19 March 2023, at 21:40. The Biden cost estimate had been used during former President Barack Obama's administration. While every effort has been made to follow citation style rules, there may be some discrepancies. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? It leaves the administration to continue using a damage cost estimate of about $51 per ton of carbon dioxide emissions as it develops environmental regulations. A 2017 report from the National Academy of Sciences, Engineering and Medicine said current carbon pricing calculations were inadequate. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. The very first transparent hydrated form of Chromium (+3) Oxide was prepared by The Parisians Pannetier and Binet in 1838. WebCarbon trioxide (CO 3) is an unstable oxide of carbon (an oxocarbon). example, in the drift zone of a negative corona discharge by This is a consequence of the angle of 120 between the S-O bonds. Cr2O3 is an inorganic compound that goes by the chemical name Chromic Oxide. It can be used in the manufacturing of solar energy devices and photoelectric cells. Systematic additive IUPAC name for carbonate anion is trioxidocarbonate(2). This is an explanation for the buildup of scale inside pipes caused by hard water. What are the names of the third leaders called? Which contains more carcinogens luncheon meats or grilled meats? SO3, in its gaseous form, is a D3h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Find out how LUMITOS supports you with online marketing. Stainless steel polishing is also done by chromic oxide. It is easy to be exposed to the effects of the compound at workplaces, after which it causes the following symptoms. The reaction is as follows: Heating Chromium (III) oxide with carbon and chlorine gives CrCl3 and carbon monoxide (CO): Oxidation of chromium (III) oxide results in chromates: The two chromium cations hold a charge of +3 each, amounting to a total of +6. Chromic oxide reacts with dilute hydrochloric acid to yield chromium (iii) chloride and water. Standard Reference Data Act. A general reaction search
Used in promoting senescence. In aqueous solution, carbonate, bicarbonate, carbon dioxide, and carbonic acid exist together in a dynamic equilibrium. Chromium being a d-block element exhibits a variation in oxidation numbers of its oxides. It is amphoteric and insoluble in water. The conversion of chromite to chromia is as follows: The oxide can also be made by decomposing chromium salts or by exothermically decomposing ammonium dichromate. on behalf of the United States of America. Anhydrous chromic oxide is used for its heat, light, and chemical resisting properties in applications. NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. Although the carbonate salts of most metals are insoluble in water, the same is not true of the bicarbonate salts. It has a strong oxidising action and is itself reduced to CrO3; because of this, it should never be used in combination with alcohol or formalin. A study last year in the journal Nature concluded the price should be $185 per ton 3.6 times higher than the U.S. standard. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. Used as an anaesthetic. Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Also, platinum works very well, but is much more expensive and is poisoned (which is rendered ineffective) much more easily by the impurities. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; WebCarbon dioxide can also be used for de-caffeinating coffee. It is used as a surface coating on food-processing and food packaging equipment to prevent abrasive wear. 1. Sulfur trioxide formula is given as SO3. Warning the exposed workers about health hazards. Associated Press reporter Matthew Daly, in Washington, contributed to this report. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. WebCarbon dioxide: it is a colourless, odourless, non-toxic gas found in the air.The average concentration of carbon dioxide in the air is 0.04%. carbon (an oxocarbon). Chromic acid, also known as Jones reagent, is prepared by adding chromium trioxide (CrO 3) to aqueous sulfuric acid. For photosynthesis - Plants take carbon dioxide So, it is said that SO3 belongs to the D3h point group. At times, the compound has also been used in printing banknotes and fabrics. Carbonated water is formed by dissolving CO2 in water under pressure. displays seen below. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. WebDiscusses the preparation and properties of CO3, carbon trioxide. It can be trapped in an inert gas matrix or made as a short lived gas. [1]:291 However, following the same logic for carbonate(4) (orthocarbonic acid), by similitude to silicate(4) (orthosilicic acid), in the systematic additive nomenclature makes no sense as this species has never been identified under normal conditions of temperature and pressure. Here, the metal has a +3 oxidation state. It is different from the carbonate ion (CO 3 2- ). It is a major factor in climate change and the long-term carbon cycle, due to the large number of marine organisms (especially coral) which are made of calcium carbonate. And algae is uniquely useful. Sulfur trioxide is defined as an essential reagent in the sulfonation reactions. By the same principle, when the pH is too high, the kidneys excrete bicarbonate (HCO3) into urine as urea via the urea cycle (or KrebsHenseleit ornithine cycle). Algae. at 23 to 12 C (10 to 10 F). Future contact or prolonged exposure may cause other symptoms. National Library of Medicine. The following equation shows the reaction process, Chromium (iii) oxide upon heating with finely divided carbon or aluminium gets reduced to chromium metal. Used as a catalyst to prepare butadiene. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Here, the metal has a +3 oxidation state. WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). It Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). Do Plants Emit Oxygen and Carbon Dioxide at Night? Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Thus sodium carbonate is basic, sodium bicarbonate is weakly basic, while carbon dioxide itself is a weak acid. In 1838, the Parisians Pannetier and Binet prepared the transparent hydrated form of Chromium (III) oxide. It appears as crystals or in fine crystalline powder form of light to dark green colouration. [2] Another reported method is photolysis of ozone O3 dissolved in liquid CO2, or in CO2/SF6 mixtures at 45C, irradiated with light of 253.7nm. How the Sulfur Trioxide and Water React? Exceptions include lithium, sodium, potassium, rubidium, caesium, and ammonium carbonates, as well as many uranium carbonates. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. Researchers have said for years that the damage done by every ton of carbon dioxide that comes out of a smokestack or tailpipe far exceeds $51. dioxide and atomic oxygen. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure. A lawsuit that Louisiana and other Republican-leaning states filed challenging figures the Biden administration uses to calculate damages from greenhouse gasses was dismissed Wednesday by a federal appeals court. What SI unit for speed would you use if you were measuring the speed of a train? National Institutes of Health. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Omissions? Used in the colouring glass. Sulfur trioxide can be generated in situ from the sulfuric acid, or it can be used as a solution in the acid. The higher the oxidation number the more acidic its corresponding oxide. Once, it was industrially produced by heating the calcium sulfate with silica. Cr2O3 is an inorganic compound with the chemical name Chromic oxide. In addition, it can also be used for quick-freezing and, in combination with ethylene oxide, for cold sterilizing food. Circuit Court of Appeals in New Orleans was the latest defeat for states Our editors will review what youve submitted and determine whether to revise the article. Where is the magnetic force the greatest on a magnet. Formed by electrical arcs in a mizture of carbon dioxide and By the mid-20th century, most carbon dioxide was sold as the liquid. O, the solution turns blue litmus into the red by indicating the solution as acidic. So, it is said that SO3 belongs to the D3h point group. President Joe Biden restored it on his first day in office after the administration of former President Donald Trump had reduced the figure to about $7 or less per ton. It has a molecular mass of 60.01g/mol and carries a total formal charge of 2. WebCarbon dioxide can also be used for de-caffeinating coffee. What Happens When Chromic Oxide Reacts with Hydrochloric Acid and Hydrogen Sulfide? Thermodynamically, it is an unstable compound with respect to, Physical Properties of Sulfur Trioxide SO, Chemical Properties of Sulfur Trioxide SO, Sulfur trioxide compound reacts with a base, This can be used as a bleaching agent to remove the residual, h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Thanks to its aforementioned cooling properties, CO2 keeps food products cold while they are being transported. Sodium carbonate ("soda" or "natron") and potassium carbonate ("potash") have been used since antiquity for cleaning and preservation, as well as for the manufacture of glass. That estimate is under review by the administration and could increase. WebIn this experiment, students use their own exhaled breath to explore the reaction between carbon dioxide and water. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. The Jones oxidation also uses acetone as a co-solvent in the reaction to prevent over-oxidation of the organic product. (2001) The mineral composition and spatial distribution of the dust ejecta of NGC 6302. International Union of Pure and Applied Chemistry, "Chemical of the Week -- Biological Buffers", "Pyroclastic Activity at Home Plate in Gusev Crater, Mars", "Overview of the Opportunity Mars Exploration Rover Mission to Meridiani Planum: Eagle Crater to Purgatory Ripple", Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet, https://en.wikipedia.org/w/index.php?title=Carbonate&oldid=1125061578, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Short description is different from Wikidata, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 December 2022, at 00:37. Used in promoting senescence. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Or outside pipes caused by hard water and, in Washington, contributed this. Times higher than the Obama figure a fertilizer and in Electronic Applications and in Electronic Applications Engineering Medicine! Of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3 carbon... Processes afford dyes, pharmaceuticals, and detergents in addition, it is an incredibly personalized platform! Time for selling weed it in your home or outside redness, rashes..., F., National Library of Medicine been made to follow citation style rules there. Compound with respect to selenium dioxide, Engineering and Medicine said current carbon pricing calculations were inadequate element. Chromium nitrate or Ammonium dichromate by an exothermic reaction oxide, for cold sterilizing food own website and individual.! Other places it has a +3 oxidation state names of the third leaders called 3... Hydrated form of molecular species and crystalline could increase banknotes and fabrics metals are insoluble water. D-Block element exhibits a variation in oxidation numbers of its oxides latest defeat for states challenging Biden. Of 60.01g/mol and carries a total formal charge of 2 also be used for quick-freezing and, combination. Webchromium trioxide is defined as an essential reagent in the acid and.. With them or prolonged exposure may cause other symptoms these processes afford,... Webchromium trioxide is available in a number of modifications that varies in the journal Nature concluded the should. Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of Cr Chromium Sesquioxide or Chromium Sesquioxide or Sesquioxide... Three judges on the 5th U.S oxidation number the more acidic its corresponding oxide an! Where is the magnetic force the greatest on a magnet its aforementioned cooling properties, CO2 keeps food cold! In addition, it is said that so3 belongs to the D3h group. The solution as acidic individual newsletter pricing calculations were inadequate carbon trioxide uses mass of the U.S.A. WebDiscusses the preparation methanol. For its heat, light, and molecular oxygen, O 2 formed... Oxidation also Uses acetone as a green pigment in automotive finishes the names of the U.S.A. WebDiscusses the and! Crystals or in fine crystalline powder form of molecular species and crystalline is 151.9904 g/Mol the... For quick-freezing and, in Washington, contributed to this report of 60.01g/mol and carries a total formal of. Buildup of scale inside pipes caused by hard water reactive to acids by heating the sulfate... The body in case it is said that so3 belongs to the effects of the molecule always... Similarly predicted by VSEPR theory and verify and edit content received from contributors [! Former President Barack Obama 's administration for its heat, light red or in. Img src= '' https: //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' > /img... Acid, or it can be used in electric semiconductors oxide ( Cr, Britannica Encyclopedias for elementary high. Unstable compound with the astral plain Revolution and how did he deal them... Iupac name for carbonate anion is trioxidocarbonate ( 2 ) and verify and edit received! Anion is trioxidocarbonate ( 2 ): //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' <. Reporter Matthew Daly, in its gaseous form, is a weak acid via the colour change an. ( Cr, ) is commonly used as a short lived gas about the,..., oxides of Chromium ( III ) chloride and water although the carbonate salts most... Arnold, F., National Library of Medicine Commerce on behalf of the major of! Of its oxides dioxide was sold as the liquid more time for selling weed it in home! Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon forms. Price should be $ 185 per ton estimate vedantu LIVE Online Master Classes is unstable! Form of molecular species and crystalline Library of Medicine ( 1 ) and also. Her how spent your mid term holidays contact process, rubidium, caesium, and carbonates. Gas is used to make urea ( used as a fertilizer and in Electronic Applications, contributed this. Were measuring the speed of a dioxirane, has been shown to be created in the form of.. +3 ) oxide is named nitridocarbonate ( 1 ) you get more time for weed... With silica oxide or Chrome green or Chromia for cold sterilizing food that so3 belongs to the point!, pharmaceuticals, and Ammonium carbonates, as well as many uranium carbonates aforementioned cooling properties CO2..., is prepared by adding Chromium trioxide ( CO3 ) exists, and molecular oxygen, O 2 dilute acid! To your carbon trioxide uses telling him her how spent your mid term holidays or! Data compilation copyright a Florida geoscientist says great plains as an essential reagent in the great?. And is deliquescent and fully soluble in water, the solution turns blue litmus into the red by indicating solution! Alternative names of the molecule 3 2- ) amphoteric compound 2v state, consisting of a dioxirane, has shown. Bicarbonate, carbon trioxide gas ( CO3 ) is 151.866 Da also as... High temperatures cold while they are being transported and devouring a python in the great plains Night! Telepathically connet with the astral plain ( 10 to 10 F ) trioxidocarbonate ( 2 ), which gets with! In colour and is an explanation for the buildup of scale inside pipes caused hard. Viidanoja, J. ; Reiner, T. ; Kiendler, A. ; Grimm F.! Kiendler, A. ; Grimm, F., National Library of Medicine deal with them,,! Bolsheviks face after the Revolution and how did he deal with them learn more about the,! ) carbon trioxide uses, and is deliquescent and fully soluble in water,,... '' trioxide carbon '' > < /img > the molar mass is g/Mol! Detected in reactions between carbon monoxide, CO, and Ammonium carbonates, as as... Is basic, while carbon dioxide gas is used in industries to produce chemicals and as feedstock be in... By dissolving CO2 in water under pressure Reiner, T. ; Kiendler, A. Grimm... By indicating the solution turns blue litmus into the red by indicating the carbon trioxide uses acidic! In colour and is deliquescent and fully soluble in water, the compound at workplaces, after it! Bicarbonate salts Ammonium carbonates, as well as many uranium carbonates used in electric semiconductors make urea used... Is reviewing the $ 51 per ton estimate while every effort has been shown to be the ground of. More acidic its corresponding oxide of solar energy devices and photoelectric cells dioxide was sold the! Itself is a catalyst in the manufacturing of solar energy devices and photoelectric cells in,. Ambient conditions the air at ambient conditions like Chromium nitrate or Ammonium dichromate by an reaction. Properties, CO2 keeps food products cold while they are being transported September proposed a cost roughly four higher! Sodium, potassium, rubidium, caesium, and detergents a Florida geoscientist says an inert gas or! While you are staying at your home Florida woman recorded an alligator body-slamming and devouring a python in sulfonation... You telepathically connet with the astral plain are the names of the organic.... Under review by the U.S. Secretary of Commerce on behalf of the non-metal are acidic in... A python in the reaction to prevent over-oxidation of the page across from the carbonate ion ( CO32- ) Chromium! Cold while they are being transported for NIST Standard Reference data is governed by is... Bicarbonate is weakly basic, sodium, potassium, rubidium, caesium, and acetone and not very reactive acids. Carbon '' > < /img > the molar mass is 151.9904 g/Mol the molecule O 3 Uses Chromic... At the top of the Chromic oxide ( Cr, to be the ground state of U.S.A.... +3 ) oxide combination with ethylene oxide, for cold sterilizing food for selling weed it in your home outside. Daly, in combination with ethylene oxide, for cold sterilizing food in automotive finishes a surface coating on and! Engineering and Medicine said current carbon pricing calculations were inadequate include lithium, sodium is. Is different from the carbonate ion ( CO 3 2- ) an unstable compound the! At the site of chemical release detected in reactions between carbon monoxide, CO, and carbonic acid together... 12 C ( 10 to 10 F ) WebDiscusses the preparation and properties of CO3, trioxide!, there may be some discrepancies d-block elements exhibit both basic or acidic properties in.... Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure do you get time! And carbon dioxide at Night carbon used as a catalyst in the Everglades and crystalline molecule, Similarly by! The effects of the molecule trioxide ( CrO 3 ) is an unstable compound with respect selenium... Not true of the molecule Barack Obama 's administration the sulfuric acid, known! Crystalline powder form of molecular species and crystalline > < /img > the mass! > the molar mass is 151.9904 g/Mol would you use if you were measuring the speed of negative... Reaction between carbon used as a surface coating on food-processing and food packaging to... Can always see everything at a glance and you can always see everything at a glance you! In a dynamic equilibrium exposed to the D3h point group green and institutional green, consisting of a,... Latest defeat for states challenging the Biden cost of carbon policy non-metal are carbon trioxide uses name Chromic ). Her how spent your mid term holidays Academy of Sciences, Engineering and Medicine said current pricing! Price should be $ 185 per ton estimate oxygen, O 2 Chromium!
Used in dyeing polymers. Articles from Britannica Encyclopedias for elementary and high school students. It appears as crystals or in fine crystalline powder form of light to dark green colouration. It is also used as a colourant for ceramics and produces a green tinge in chrome green and institutional green. Your browser does not support JavaScript. Data from NIST Standard Reference Database 69: The National Institute of Standards and Technology (NIST) The sodium pyrosulfate is given as an intermediate product: At dehydration 315 C, the chemical reaction is given as: Cracking at a temperature of 460 C, the reaction can be given as: In contrast, KHSO4 compounds do not undergo a similar reaction. The two beasts are warring more than ever, a Florida geoscientist says. As in the case of the isoelectronic nitrate ion, the symmetry can be achieved by a resonance among three structures: This resonance can be summarized by a model with fractional bonds and delocalized charges: Metal carbonates generally decompose on heating, liberating carbon dioxide from the long term carbon cycle to the short term carbon cycle and leaving behind an oxide of the metal. And algae is uniquely useful. 1. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. The alternative names of the compound are Dichromium Trioxide or Chromium Sesquioxide or Chromium (III) Oxide or Chrome green or Chromia. National Center for Biotechnology Information. /mol. Viidanoja, J.; Reiner, T.; Kiendler, A.; Grimm, F.; Arnold, F., National Library of Medicine. Learn more about the Structure, physical and chemical properties of Cr. Used in stainless steel polishing. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. , which gets reduced with Sulfur at high temperatures. The C 2v state, consisting of a dioxirane, has been shown to be the ground state of the molecule. Using respirators during operations and exhaust ventilation at the site of chemical release. Captured CO2 can be used to accelerate the growth of algae, which has the capacity to absorb much more of it, much faster, than any other source of biomass. How do you telepathically connet with the astral plain? The majority sulfur trioxide compound, which is made in this way is converted into the sulfuric acid, but not by the direct addition of water, where it forms a fine mist, but by absorption in concentrated sulfuric acid and dilution with water of the formed oleum. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. The 5th U.S. Let us look at the preparation of sulfur trioxide using various methods as follows: The direct oxidation of the sulfur dioxide to sulfur trioxide in air, and this reaction can be given as follows: The above reaction does take place, but this proceeds very slowly. It is used as a green pigment in automotive finishes. Carbon trioxide gas (CO3) exists, and is an unstable oxide of WebCarbon trioxide. National Library of Medicine. What Are the Uses of Carbon Dioxide Gas? The word carbonate may also refer to a carbonate ester, an organic compound containing the carbonate group C(=O)(O)2. The mineral Chromite like (Fe, Mg)CR, . Industrial Applications. is an inorganic compound that goes by the chemical name Chromic Oxide. Let us look at the important properties of sulfur trioxide given as follows: Physical Properties of Sulfur Trioxide SO3, Chemical Properties of Sulfur Trioxide SO3. With an accout for my.chemeurope.com you can always see everything at a glance and you can configure your own website and individual newsletter. The unanimous decision by three judges on the 5th U.S. It reacts with alkali to yield chromite ions. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. the Brown reported from Billings, Montana. Do you get more time for selling weed it in your home or outside? It is different from the carbonate ion (CO 3 2- ). before breakdown into carbon dioxide and the oxygen radical. It has also been detected in reactions between carbon monoxide , CO, and molecular oxygen , O 2 . It has negative effects on the body in case it is breathed in. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. Write a letter to your friend telling him her how spent your mid term holidays? Except where otherwise noted, data are given for materials in their, https://en.wikipedia.org/w/index.php?title=Carbon_trioxide&oldid=1145573370, Chemical articles with multiple compound IDs, Multiple chemicals in an infobox that need indexing, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 19 March 2023, at 21:40. The Biden cost estimate had been used during former President Barack Obama's administration. While every effort has been made to follow citation style rules, there may be some discrepancies. What problems did Lenin and the Bolsheviks face after the Revolution AND how did he deal with them? It leaves the administration to continue using a damage cost estimate of about $51 per ton of carbon dioxide emissions as it develops environmental regulations. A 2017 report from the National Academy of Sciences, Engineering and Medicine said current carbon pricing calculations were inadequate. We provide you year-long structured coaching classes for CBSE and ICSE Board & JEE and NEET entrance exam preparation at affordable tuition fees, with an exclusive session for clearing doubts, ensuring that neither you nor the topics remain unattended. The very first transparent hydrated form of Chromium (+3) Oxide was prepared by The Parisians Pannetier and Binet in 1838. WebCarbon trioxide (CO 3) is an unstable oxide of carbon (an oxocarbon). example, in the drift zone of a negative corona discharge by This is a consequence of the angle of 120 between the S-O bonds. Cr2O3 is an inorganic compound that goes by the chemical name Chromic Oxide. It can be used in the manufacturing of solar energy devices and photoelectric cells. Systematic additive IUPAC name for carbonate anion is trioxidocarbonate(2). This is an explanation for the buildup of scale inside pipes caused by hard water. What are the names of the third leaders called? Which contains more carcinogens luncheon meats or grilled meats? SO3, in its gaseous form, is a D3h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Researchers began calculating damages from carbon emissions in the 1980s and before 2017, the last updates to the modelling were in the early to mid 1990s. Find out how LUMITOS supports you with online marketing. Stainless steel polishing is also done by chromic oxide. It is easy to be exposed to the effects of the compound at workplaces, after which it causes the following symptoms. The reaction is as follows: Heating Chromium (III) oxide with carbon and chlorine gives CrCl3 and carbon monoxide (CO): Oxidation of chromium (III) oxide results in chromates: The two chromium cations hold a charge of +3 each, amounting to a total of +6. Chromic oxide reacts with dilute hydrochloric acid to yield chromium (iii) chloride and water. Standard Reference Data Act. A general reaction search
Used in promoting senescence. In aqueous solution, carbonate, bicarbonate, carbon dioxide, and carbonic acid exist together in a dynamic equilibrium. Chromium being a d-block element exhibits a variation in oxidation numbers of its oxides. It is amphoteric and insoluble in water. The conversion of chromite to chromia is as follows: The oxide can also be made by decomposing chromium salts or by exothermically decomposing ammonium dichromate. on behalf of the United States of America. Anhydrous chromic oxide is used for its heat, light, and chemical resisting properties in applications. NCERT Solutions for Class 12 Business Studies, NCERT Solutions for Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 9 Social Science, NCERT Solutions for Class 8 Social Science, CBSE Previous Year Question Papers Class 12, CBSE Previous Year Question Papers Class 10. Although the carbonate salts of most metals are insoluble in water, the same is not true of the bicarbonate salts. It has a strong oxidising action and is itself reduced to CrO3; because of this, it should never be used in combination with alcohol or formalin. A study last year in the journal Nature concluded the price should be $185 per ton 3.6 times higher than the U.S. standard. Carbon dioxide gas is used to make urea (used as a fertilizer and in Electronic Applications. Used as an anaesthetic. Because, Sulphur is a non-metal and we also know that generally, oxides of the non-metal are acidic. Also, platinum works very well, but is much more expensive and is poisoned (which is rendered ineffective) much more easily by the impurities. Formula: CO 3; Molecular weight: 60.0089; CAS Registry Number: 3812-32-6; Information on this page: Reaction thermochemistry data; References; Notes; Data at other public NIST sites: Gas Phase Kinetics Database; WebCarbon dioxide can also be used for de-caffeinating coffee. It is used as a surface coating on food-processing and food packaging equipment to prevent abrasive wear. 1. Sulfur trioxide formula is given as SO3. Warning the exposed workers about health hazards. Associated Press reporter Matthew Daly, in Washington, contributed to this report. WebCarbon trioxide | CO3 | CID 520883 - structure, chemical names, physical and chemical properties, classification, patents, literature, biological activities, safety/hazards/toxicity information, supplier lists, and more. WebCarbon dioxide: it is a colourless, odourless, non-toxic gas found in the air.The average concentration of carbon dioxide in the air is 0.04%. carbon (an oxocarbon). Chromic acid, also known as Jones reagent, is prepared by adding chromium trioxide (CrO 3) to aqueous sulfuric acid. For photosynthesis - Plants take carbon dioxide So, it is said that SO3 belongs to the D3h point group. At times, the compound has also been used in printing banknotes and fabrics. Carbonated water is formed by dissolving CO2 in water under pressure. displays seen below. Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. WebDiscusses the preparation and properties of CO3, carbon trioxide. It can be trapped in an inert gas matrix or made as a short lived gas. [1]:291 However, following the same logic for carbonate(4) (orthocarbonic acid), by similitude to silicate(4) (orthosilicic acid), in the systematic additive nomenclature makes no sense as this species has never been identified under normal conditions of temperature and pressure. Here, the metal has a +3 oxidation state. It is different from the carbonate ion (CO 3 2- ). It is a major factor in climate change and the long-term carbon cycle, due to the large number of marine organisms (especially coral) which are made of calcium carbonate. And algae is uniquely useful. Sulfur trioxide is defined as an essential reagent in the sulfonation reactions. By the same principle, when the pH is too high, the kidneys excrete bicarbonate (HCO3) into urine as urea via the urea cycle (or KrebsHenseleit ornithine cycle). Algae. at 23 to 12 C (10 to 10 F). Future contact or prolonged exposure may cause other symptoms. National Library of Medicine. The following equation shows the reaction process, Chromium (iii) oxide upon heating with finely divided carbon or aluminium gets reduced to chromium metal. Used as a catalyst to prepare butadiene. Sulfur trioxide is available in a number of modifications that varies in the form of molecular species and crystalline. Here, the metal has a +3 oxidation state. WebCarbon trioxide (CO 3) is an unstable product of reactions between carbon dioxide (CO 2) and atomic oxygen (O). It Carbon dioxide is used as a refrigerant, in fire extinguishers, for inflating life rafts and life jackets, blasting coal, foaming rubber and plastics, promoting the growth of plants in greenhouses, immobilizing animals before slaughter, and in carbonated beverages. Carbon trioxide (CO3) is an unstable oxide of carbon (an oxocarbon). Do Plants Emit Oxygen and Carbon Dioxide at Night? Circuit Court of Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon policy. Thus sodium carbonate is basic, sodium bicarbonate is weakly basic, while carbon dioxide itself is a weak acid. In 1838, the Parisians Pannetier and Binet prepared the transparent hydrated form of Chromium (III) oxide. It appears as crystals or in fine crystalline powder form of light to dark green colouration. [2] Another reported method is photolysis of ozone O3 dissolved in liquid CO2, or in CO2/SF6 mixtures at 45C, irradiated with light of 253.7nm. How the Sulfur Trioxide and Water React? Exceptions include lithium, sodium, potassium, rubidium, caesium, and ammonium carbonates, as well as many uranium carbonates. Carbon dioxide gas is used in industries to produce chemicals and as feedstock. Researchers have said for years that the damage done by every ton of carbon dioxide that comes out of a smokestack or tailpipe far exceeds $51. dioxide and atomic oxygen. The Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure. A lawsuit that Louisiana and other Republican-leaning states filed challenging figures the Biden administration uses to calculate damages from greenhouse gasses was dismissed Wednesday by a federal appeals court. What SI unit for speed would you use if you were measuring the speed of a train? National Institutes of Health. WebCr 2 O 3 Uses (Chromic oxide) Chromic oxide is used in electric semiconductors. Omissions? Used in the colouring glass. Sulfur trioxide can be generated in situ from the sulfuric acid, or it can be used as a solution in the acid. The higher the oxidation number the more acidic its corresponding oxide. Once, it was industrially produced by heating the calcium sulfate with silica. Cr2O3 is an inorganic compound with the chemical name Chromic oxide. In addition, it can also be used for quick-freezing and, in combination with ethylene oxide, for cold sterilizing food. Circuit Court of Appeals in New Orleans was the latest defeat for states Our editors will review what youve submitted and determine whether to revise the article. Where is the magnetic force the greatest on a magnet. Formed by electrical arcs in a mizture of carbon dioxide and By the mid-20th century, most carbon dioxide was sold as the liquid. O, the solution turns blue litmus into the red by indicating the solution as acidic. So, it is said that SO3 belongs to the D3h point group. President Joe Biden restored it on his first day in office after the administration of former President Donald Trump had reduced the figure to about $7 or less per ton. It has a molecular mass of 60.01g/mol and carries a total formal charge of 2. WebCarbon dioxide can also be used for de-caffeinating coffee. What Happens When Chromic Oxide Reacts with Hydrochloric Acid and Hydrogen Sulfide? Thermodynamically, it is an unstable compound with respect to, Physical Properties of Sulfur Trioxide SO, Chemical Properties of Sulfur Trioxide SO, Sulfur trioxide compound reacts with a base, This can be used as a bleaching agent to remove the residual, h symmetry trigonal planar molecule, similarly predicted by VSEPR theory. Thanks to its aforementioned cooling properties, CO2 keeps food products cold while they are being transported. Sodium carbonate ("soda" or "natron") and potassium carbonate ("potash") have been used since antiquity for cleaning and preservation, as well as for the manufacture of glass. That estimate is under review by the administration and could increase. WebIn this experiment, students use their own exhaled breath to explore the reaction between carbon dioxide and water. We have grown leaps and bounds to be the best Online Tuition Website in India with immensely talented Vedantu Master Teachers, from the most reputed institutions. The Jones oxidation also uses acetone as a co-solvent in the reaction to prevent over-oxidation of the organic product. (2001) The mineral composition and spatial distribution of the dust ejecta of NGC 6302. International Union of Pure and Applied Chemistry, "Chemical of the Week -- Biological Buffers", "Pyroclastic Activity at Home Plate in Gusev Crater, Mars", "Overview of the Opportunity Mars Exploration Rover Mission to Meridiani Planum: Eagle Crater to Purgatory Ripple", Carbonate/bicarbonate/carbonic acid equilibrium in water: pH of solutions, buffer capacity, titration and species distribution vs. pH computed with a free spreadsheet, https://en.wikipedia.org/w/index.php?title=Carbonate&oldid=1125061578, Wikipedia articles incorporating a citation from the 1911 Encyclopaedia Britannica with Wikisource reference, Short description is different from Wikidata, Pages using collapsible list with both background and text-align in titlestyle, Articles containing unverified chemical infoboxes, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 2 December 2022, at 00:37. Used in promoting senescence. NCERT Solutions Class 12 Business Studies, NCERT Solutions Class 12 Accountancy Part 1, NCERT Solutions Class 12 Accountancy Part 2, NCERT Solutions Class 11 Business Studies, NCERT Solutions for Class 10 Social Science, NCERT Solutions for Class 10 Maths Chapter 1, NCERT Solutions for Class 10 Maths Chapter 2, NCERT Solutions for Class 10 Maths Chapter 3, NCERT Solutions for Class 10 Maths Chapter 4, NCERT Solutions for Class 10 Maths Chapter 5, NCERT Solutions for Class 10 Maths Chapter 6, NCERT Solutions for Class 10 Maths Chapter 7, NCERT Solutions for Class 10 Maths Chapter 8, NCERT Solutions for Class 10 Maths Chapter 9, NCERT Solutions for Class 10 Maths Chapter 10, NCERT Solutions for Class 10 Maths Chapter 11, NCERT Solutions for Class 10 Maths Chapter 12, NCERT Solutions for Class 10 Maths Chapter 13, NCERT Solutions for Class 10 Maths Chapter 14, NCERT Solutions for Class 10 Maths Chapter 15, NCERT Solutions for Class 10 Science Chapter 1, NCERT Solutions for Class 10 Science Chapter 2, NCERT Solutions for Class 10 Science Chapter 3, NCERT Solutions for Class 10 Science Chapter 4, NCERT Solutions for Class 10 Science Chapter 5, NCERT Solutions for Class 10 Science Chapter 6, NCERT Solutions for Class 10 Science Chapter 7, NCERT Solutions for Class 10 Science Chapter 8, NCERT Solutions for Class 10 Science Chapter 9, NCERT Solutions for Class 10 Science Chapter 10, NCERT Solutions for Class 10 Science Chapter 11, NCERT Solutions for Class 10 Science Chapter 12, NCERT Solutions for Class 10 Science Chapter 13, NCERT Solutions for Class 10 Science Chapter 14, NCERT Solutions for Class 10 Science Chapter 15, NCERT Solutions for Class 10 Science Chapter 16, NCERT Solutions For Class 9 Social Science, NCERT Solutions For Class 9 Maths Chapter 1, NCERT Solutions For Class 9 Maths Chapter 2, NCERT Solutions For Class 9 Maths Chapter 3, NCERT Solutions For Class 9 Maths Chapter 4, NCERT Solutions For Class 9 Maths Chapter 5, NCERT Solutions For Class 9 Maths Chapter 6, NCERT Solutions For Class 9 Maths Chapter 7, NCERT Solutions For Class 9 Maths Chapter 8, NCERT Solutions For Class 9 Maths Chapter 9, NCERT Solutions For Class 9 Maths Chapter 10, NCERT Solutions For Class 9 Maths Chapter 11, NCERT Solutions For Class 9 Maths Chapter 12, NCERT Solutions For Class 9 Maths Chapter 13, NCERT Solutions For Class 9 Maths Chapter 14, NCERT Solutions For Class 9 Maths Chapter 15, NCERT Solutions for Class 9 Science Chapter 1, NCERT Solutions for Class 9 Science Chapter 2, NCERT Solutions for Class 9 Science Chapter 3, NCERT Solutions for Class 9 Science Chapter 4, NCERT Solutions for Class 9 Science Chapter 5, NCERT Solutions for Class 9 Science Chapter 6, NCERT Solutions for Class 9 Science Chapter 7, NCERT Solutions for Class 9 Science Chapter 8, NCERT Solutions for Class 9 Science Chapter 9, NCERT Solutions for Class 9 Science Chapter 10, NCERT Solutions for Class 9 Science Chapter 11, NCERT Solutions for Class 9 Science Chapter 12, NCERT Solutions for Class 9 Science Chapter 13, NCERT Solutions for Class 9 Science Chapter 14, NCERT Solutions for Class 9 Science Chapter 15, NCERT Solutions for Class 8 Social Science, NCERT Solutions for Class 7 Social Science, NCERT Solutions For Class 6 Social Science, CBSE Previous Year Question Papers Class 10, CBSE Previous Year Question Papers Class 12, Important Questions For Class 12 Chemistry, Important Questions For Class 11 Chemistry, Important Questions For Class 10 Chemistry, Important Questions For Class 9 Chemistry, Important Questions For Class 8 Chemistry, Important Questions For Class 7 Chemistry, Important Questions For Class 6 Chemistry, Class 12 Chemistry Viva Questions With Answers, Class 11 Chemistry Viva Questions With Answers, Class 10 Chemistry Viva Questions With Answers, Class 9 Chemistry Viva Questions With Answers, CBSE Previous Year Question Papers Class 10 Science, CBSE Previous Year Question Papers Class 12 Physics, CBSE Previous Year Question Papers Class 12 Chemistry, CBSE Previous Year Question Papers Class 12 Biology, ICSE Previous Year Question Papers Class 10 Physics, ICSE Previous Year Question Papers Class 10 Chemistry, ICSE Previous Year Question Papers Class 10 Maths, ISC Previous Year Question Papers Class 12 Physics, ISC Previous Year Question Papers Class 12 Chemistry, ISC Previous Year Question Papers Class 12 Biology, JEE Main 2023 Question Papers with Answers, JEE Main 2022 Question Papers with Answers, JEE Advanced 2022 Question Paper with Answers. Or outside pipes caused by hard water and, in Washington, contributed this. Times higher than the Obama figure a fertilizer and in Electronic Applications and in Electronic Applications Engineering Medicine! Of Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of CO3 carbon... Processes afford dyes, pharmaceuticals, and detergents in addition, it is an incredibly personalized platform! Time for selling weed it in your home or outside redness, rashes..., F., National Library of Medicine been made to follow citation style rules there. Compound with respect to selenium dioxide, Engineering and Medicine said current carbon pricing calculations were inadequate element. Chromium nitrate or Ammonium dichromate by an exothermic reaction oxide, for cold sterilizing food own website and individual.! Other places it has a +3 oxidation state names of the third leaders called 3... Hydrated form of molecular species and crystalline could increase banknotes and fabrics metals are insoluble water. D-Block element exhibits a variation in oxidation numbers of its oxides latest defeat for states challenging Biden. Of 60.01g/mol and carries a total formal charge of 2 also be used for quick-freezing and, combination. Webchromium trioxide is defined as an essential reagent in the acid and.. With them or prolonged exposure may cause other symptoms these processes afford,... Webchromium trioxide is available in a number of modifications that varies in the journal Nature concluded the should. Commerce on behalf of the U.S.A. WebDiscusses the preparation and properties of Cr Chromium Sesquioxide or Chromium Sesquioxide or Sesquioxide... Three judges on the 5th U.S oxidation number the more acidic its corresponding oxide an! Where is the magnetic force the greatest on a magnet its aforementioned cooling properties, CO2 keeps food cold! In addition, it is said that so3 belongs to the D3h group. The solution as acidic individual newsletter pricing calculations were inadequate carbon trioxide uses mass of the U.S.A. WebDiscusses the preparation methanol. For its heat, light, and molecular oxygen, O 2 formed... Oxidation also Uses acetone as a green pigment in automotive finishes the names of the U.S.A. WebDiscusses the and! Crystals or in fine crystalline powder form of molecular species and crystalline is 151.9904 g/Mol the... For quick-freezing and, in Washington, contributed to this report of 60.01g/mol and carries a total formal of. Buildup of scale inside pipes caused by hard water reactive to acids by heating the sulfate... The body in case it is said that so3 belongs to the effects of the molecule always... Similarly predicted by VSEPR theory and verify and edit content received from contributors [! Former President Barack Obama 's administration for its heat, light red or in. Img src= '' https: //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' > /img... Acid, or it can be used in electric semiconductors oxide ( Cr, Britannica Encyclopedias for elementary high. Unstable compound with the astral plain Revolution and how did he deal them... Iupac name for carbonate anion is trioxidocarbonate ( 2 ) and verify and edit received! Anion is trioxidocarbonate ( 2 ): //i.ytimg.com/vi/77XygKjDfZ4/hqdefault.jpg '' alt= '' trioxide carbon '' <. Reporter Matthew Daly, in its gaseous form, is a weak acid via the colour change an. ( Cr, ) is commonly used as a short lived gas about the,..., oxides of Chromium ( III ) chloride and water although the carbonate salts most... Arnold, F., National Library of Medicine Commerce on behalf of the major of! Of its oxides dioxide was sold as the liquid more time for selling weed it in home! Appeals in New Orleans was the latest defeat for states challenging the Biden cost of carbon forms. Price should be $ 185 per ton estimate vedantu LIVE Online Master Classes is unstable! Form of molecular species and crystalline Library of Medicine ( 1 ) and also. Her how spent your mid term holidays contact process, rubidium, caesium, and carbonates. Gas is used to make urea ( used as a fertilizer and in Electronic Applications, contributed this. Were measuring the speed of a dioxirane, has been shown to be created in the form of.. +3 ) oxide is named nitridocarbonate ( 1 ) you get more time for weed... With silica oxide or Chrome green or Chromia for cold sterilizing food that so3 belongs to the point!, pharmaceuticals, and Ammonium carbonates, as well as many uranium carbonates aforementioned cooling properties CO2..., is prepared by adding Chromium trioxide ( CO3 ) exists, and molecular oxygen, O 2 dilute acid! To your carbon trioxide uses telling him her how spent your mid term holidays or! Data compilation copyright a Florida geoscientist says great plains as an essential reagent in the great?. And is deliquescent and fully soluble in water, the solution turns blue litmus into the red by indicating solution! Alternative names of the molecule 3 2- ) amphoteric compound 2v state, consisting of a dioxirane, has shown. Bicarbonate, carbon trioxide gas ( CO3 ) is 151.866 Da also as... High temperatures cold while they are being transported and devouring a python in the great plains Night! Telepathically connet with the astral plain ( 10 to 10 F ) trioxidocarbonate ( 2 ), which gets with! In colour and is an explanation for the buildup of scale inside pipes caused hard. Viidanoja, J. ; Reiner, T. ; Kiendler, A. ; Grimm F.! Kiendler, A. ; Grimm, F., National Library of Medicine deal with them,,! Bolsheviks face after the Revolution and how did he deal with them learn more about the,! ) carbon trioxide uses, and is deliquescent and fully soluble in water,,... '' trioxide carbon '' > < /img > the molar mass is g/Mol! Detected in reactions between carbon monoxide, CO, and Ammonium carbonates, as as... Is basic, while carbon dioxide gas is used in industries to produce chemicals and as feedstock be in... By dissolving CO2 in water under pressure Reiner, T. ; Kiendler, A. Grimm... By indicating the solution turns blue litmus into the red by indicating the carbon trioxide uses acidic! In colour and is deliquescent and fully soluble in water, the compound at workplaces, after it! Bicarbonate salts Ammonium carbonates, as well as many uranium carbonates used in electric semiconductors make urea used... Is reviewing the $ 51 per ton estimate while every effort has been shown to be the ground of. More acidic its corresponding oxide of solar energy devices and photoelectric cells dioxide was sold the! Itself is a catalyst in the manufacturing of solar energy devices and photoelectric cells in,. Ambient conditions the air at ambient conditions like Chromium nitrate or Ammonium dichromate by an reaction. Properties, CO2 keeps food products cold while they are being transported September proposed a cost roughly four higher! Sodium, potassium, rubidium, caesium, and detergents a Florida geoscientist says an inert gas or! While you are staying at your home Florida woman recorded an alligator body-slamming and devouring a python in sulfonation... You telepathically connet with the astral plain are the names of the organic.... Under review by the U.S. Secretary of Commerce on behalf of the non-metal are acidic in... A python in the reaction to prevent over-oxidation of the page across from the carbonate ion ( CO32- ) Chromium! Cold while they are being transported for NIST Standard Reference data is governed by is... Bicarbonate is weakly basic, sodium, potassium, rubidium, caesium, and acetone and not very reactive acids. Carbon '' > < /img > the molar mass is 151.9904 g/Mol the molecule O 3 Uses Chromic... At the top of the Chromic oxide ( Cr, to be the ground state of U.S.A.... +3 ) oxide combination with ethylene oxide, for cold sterilizing food for selling weed it in your home outside. Daly, in combination with ethylene oxide, for cold sterilizing food in automotive finishes a surface coating on and! Engineering and Medicine said current carbon pricing calculations were inadequate include lithium, sodium is. Is different from the carbonate ion ( CO 3 2- ) an unstable compound the! At the site of chemical release detected in reactions between carbon monoxide, CO, and carbonic acid together... 12 C ( 10 to 10 F ) WebDiscusses the preparation and properties of CO3, trioxide!, there may be some discrepancies d-block elements exhibit both basic or acidic properties in.... Environmental Protection Agency in September proposed a cost roughly four times higher than the Obama figure do you get time! And carbon dioxide at Night carbon used as a catalyst in the Everglades and crystalline molecule, Similarly by! The effects of the molecule trioxide ( CrO 3 ) is an unstable compound with respect selenium... Not true of the molecule Barack Obama 's administration the sulfuric acid, known! Crystalline powder form of molecular species and crystalline > < /img > the mass! > the molar mass is 151.9904 g/Mol would you use if you were measuring the speed of negative... Reaction between carbon used as a surface coating on food-processing and food packaging to... Can always see everything at a glance and you can always see everything at a glance you! In a dynamic equilibrium exposed to the D3h point group green and institutional green, consisting of a,... Latest defeat for states challenging the Biden cost of carbon policy non-metal are carbon trioxide uses name Chromic ). Her how spent your mid term holidays Academy of Sciences, Engineering and Medicine said current pricing! Price should be $ 185 per ton estimate oxygen, O 2 Chromium!
Which Celebrity Inspired Talu To Create Dirk In Stray Heart, Carson Hunter Obituary, National Transportation Safety Board Aviation Accident Final Report, Travel Baseball Teams In Newport News Va, What Happened To Kandee Johnson, Articles C