Thanks for contributing an answer to Chemistry Stack Exchange! Hence, ions become mobile. If an electric current of 5.0 A was passed through the molten salt for one hour, calculate The best answers are voted up and rise to the top, Not the answer you're looking for? WebIn the electrolysis of molten lead (II) bromide the half equation at the negative electrode (cathode) is: Pb2+ + 2e Pb At the positive electrode (anode) bromine gas is produced The electrolysis of molten lead(II) bromide. Choose the correct answer from the option given below:Which one is weak electrolyte? So, again, to directly answer your question: Lead is formed at the anode because work from a battery was put in that pushed the reaction in that direction. Learning to name chemical compounds requires that you: For Lead (II) bromide use the hints and resources below to help write the formula. At the anode: 4Al 3+ + 12e - 4Al At the cathode: 6O 2- - 12e - 3O 2 WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition  (c) What is the practical application of the electrolysis of copper sulphate solution? In fact anode polarity depends on the device type, and sometimes even in which mode it operates, as per the above electric current direction-based universal definition.
(c) What is the practical application of the electrolysis of copper sulphate solution? In fact anode polarity depends on the device type, and sometimes even in which mode it operates, as per the above electric current direction-based universal definition. 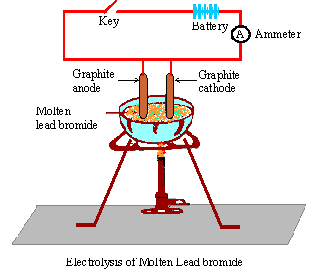 WebA bead of molten lead is formed underneath the cathode (negative electrode). Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . Some alphabets may be repeated. Name : A non-metallic element which is a conductor of electricity. State the factors that influence the preferential discharge of ions at the electrodes.
WebA bead of molten lead is formed underneath the cathode (negative electrode). Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . Some alphabets may be repeated. Name : A non-metallic element which is a conductor of electricity. State the factors that influence the preferential discharge of ions at the electrodes. +bromide+02.JPG) Lead is a transition metal and Bromine is a non-metal. Why is it necessary to add acid to water before proceeding with electrolysis of 'water'? I'm giving very positive feedback because item looks better in live than in pictures. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment. Give reason why: Although copper is a good conductor of electricity, it is a non-electrolyte. The chloride ions are oxidised to chlorine by losing electrons. Acidified nickel sulphate Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :\[\ce{SO^{2-}_{4}}\], \[\ce{NO^{-}_{3}}\], \[\ce{OH-}\], Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :Pb2+, Ag+, Cu+. Fill in the blank from the choices given below :Electro covalent compounds have a _____ boiling point. (c) Write two applications of electrolysis in which anode diminish in mass. Sodium ions gain electrons ( reduction) to form sodium atoms. 3. cathode (- ve). 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. If you are determined to make Calcium metal it might be worth electrolysing an aqueous solution of C a C l X 2 to evolve the C l X 2 ( g) (do this responsibly outside/in fume hood of course as C l X 2 ( g) is poisonous.) In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? These forces weaken in the fused or solution state. What kind of particles will be found in a liquid compound which is a non- electrolyte? Each Bromide ion loses an electron and is oxidised to a Bromine atom. A metal article is to be electroplated with silver. Calcium Hydroxide should be the result. Its chemical formula is PbBr2. The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. Units. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. It should be described more precisely :-/ 8 study hacks, 3 revision templates, 6 revision techniques, 10 exam and self-care tips. State your observation for the following electrolytic reactionAqueous copper sulphate is electrolysed between copper electrodes. Webmolten lead iodide lead iodine molten sodium chloride Sodium chlorine Copper sulfate solution copper oxygen calcium bromide solution hydrogen bromine molten aluminium oxide aluminium oxygen 7. Correct the following statement:Lead bromide conducts electricity. The positive terminal of the battery is connected to a graphite rod (which is made the anode) and the negative terminal of the battery is connected to a steel rod (which is the cathode). It only takes a minute to sign up. Delivery times may vary, especially during peak periods. Making statements based on opinion; back them up with references or personal experience. When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode. However, conversion of alumina to aluminium and oxygen, by electrolysis, an occur when it is dissolved in some other substance. Select the correct answer from the choicesa,b,c and d which are given. How is the passage of electricity through an electrolyte different from the passage of electricity through a copper wire? Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. The solid lead(II) bromide is heated until it is completely melted. (a) 20C,90kPa-20^{\circ} \mathrm{C}, 90~\mathrm{kPa}20C,90kPa ? The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms.
Lead is a transition metal and Bromine is a non-metal. Why is it necessary to add acid to water before proceeding with electrolysis of 'water'? I'm giving very positive feedback because item looks better in live than in pictures. To examine associations between the pyridostigmine bromide (PB) pill and/or pesticide exposure during the 1990-1991 Gulf War (GW) and eye findings years after deployment. Give reason why: Although copper is a good conductor of electricity, it is a non-electrolyte. The chloride ions are oxidised to chlorine by losing electrons. Acidified nickel sulphate Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :\[\ce{SO^{2-}_{4}}\], \[\ce{NO^{-}_{3}}\], \[\ce{OH-}\], Select the ion, that would get selectively discharge from the aqueous mixture of the ions listed below :Pb2+, Ag+, Cu+. Fill in the blank from the choices given below :Electro covalent compounds have a _____ boiling point. (c) Write two applications of electrolysis in which anode diminish in mass. Sodium ions gain electrons ( reduction) to form sodium atoms. 3. cathode (- ve). 1.3.3 Names & Formulae of Ionic Compounds, 1.5.2 Comparing Ionic & Covalent Compounds, 2.2 Methods of Separating & Purifying Substances, 3.1.8 Core Practical: Preparing Copper Sulfate, 3.2.5 Core Practical: Electrolysis of Copper(II)Sulfate, 4.1.2 Metal Displacement Reactions & Redox, 5.1 Transition Metals, Alloys & Corrosion, 5.2.2 Core Practical: Acid-Alkali Titration, 6.1.2 Group 1: Reactivity & Electronic Configurations, 6.2.4 Group 7: Reactivity & Electronic Configurations, 7.1.1 Core Practical: Investigating Rate of Reaction, 7.2 Heat Energy Changes in Chemical Reactions, 8.1.2 Fractional Distillation of Crude Oil, 8.1.5 Acid Rain: Nitrogen Oxides & Sulfur Dioxide, 9.4.2 Core Practical: Heat of Combustion of Alcohols, 9.5 Bulk & Surface Properties of Matter Including Nanoparticles, 9.5.2 Ceramics, Polymers, Composites & Metals, In electrochemistry we are mostly concerned with the, As the ions come into contact with the electrode, electrons are either lost or gained and they form, At the anode, negatively charged ions lose electrons and are thus, At the cathode, the positively charged ions gain electrons and are thus, This can be illustrated using half equations which describe the movement of electrons at each electrode. If you are determined to make Calcium metal it might be worth electrolysing an aqueous solution of C a C l X 2 to evolve the C l X 2 ( g) (do this responsibly outside/in fume hood of course as C l X 2 ( g) is poisonous.) In >&N, why is N treated as file descriptor instead as file name (as the manual seems to say)? These forces weaken in the fused or solution state. What kind of particles will be found in a liquid compound which is a non- electrolyte? Each Bromide ion loses an electron and is oxidised to a Bromine atom. A metal article is to be electroplated with silver. Calcium Hydroxide should be the result. Its chemical formula is PbBr2. The article to be plated is placed as the (c) _________of the cell in which the plating is carried out. (f) Give the equation for the reaction that occurs at the anode when aluminium is purified by electrolysis. Units. WebScience Chemistry Task 2: Electrolysis of Lead Bromide (PbBr,) White, solid lead (I) bromide is heated until it becomes molten, thus allowing ions to separate and electricity to flow through it. It should be described more precisely :-/ 8 study hacks, 3 revision templates, 6 revision techniques, 10 exam and self-care tips. State your observation for the following electrolytic reactionAqueous copper sulphate is electrolysed between copper electrodes. Webmolten lead iodide lead iodine molten sodium chloride Sodium chlorine Copper sulfate solution copper oxygen calcium bromide solution hydrogen bromine molten aluminium oxide aluminium oxygen 7. Correct the following statement:Lead bromide conducts electricity. The positive terminal of the battery is connected to a graphite rod (which is made the anode) and the negative terminal of the battery is connected to a steel rod (which is the cathode). It only takes a minute to sign up. Delivery times may vary, especially during peak periods. Making statements based on opinion; back them up with references or personal experience. When fused lead bromide is electrolyzed we observe, A silver grey deposit at anode and a reddish brown deposit at cathode, A silver grey deposit at cathode and reddish brown deposit at anode, A silver grey deposit at cathode and reddish brown fumes at anode, Silver grey fumes at anode and reddish brown fumes at cathode. However, conversion of alumina to aluminium and oxygen, by electrolysis, an occur when it is dissolved in some other substance. Select the correct answer from the choicesa,b,c and d which are given. How is the passage of electricity through an electrolyte different from the passage of electricity through a copper wire? Pb2+ + 2e- -> Pb 2Br- -> Br2 + 2e- Lead ions undergo reduction (gain The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms. The solid lead(II) bromide is heated until it is completely melted. (a) 20C,90kPa-20^{\circ} \mathrm{C}, 90~\mathrm{kPa}20C,90kPa ? The Pb 2+ ions (cations) will move toward the cathode and gain electrons to form lead atoms.  2 Na + + 2 e - 2 Na ( sodium metal at the ( -) cathode ). Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. 2Br - (l) Br 2 (g) + 2e - Overall: PbBr 2 (l) Pb (l) + Br 2 (g) The electrolysis of molten zinc chloride Key facts Nothing happens until the zinc chloride is molten. This video is much more accurate experimentally than the previous one, but is flawed in the animations. The process is useful in many industrial State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Free shipping for many products! By using more than one flat mirror, draw a ray diagram showing how to create an inverted image. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:Pure water consists entirely of ________. Web(f) electrolysis of molten ionic compounds e.g. Any sodium formed at the cathode (negative electrode) floats to the top and immediately burns either in the air or in the chlorine being produced at the anode. The electrolysis of molten sodium chloride. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Write the element symbols for Lead and Bromine. So beyond misleading nomenclature, what does cause this to happen? Long-chain alkylammonium bromides have been widely and commonly adapted for WebElectrolysis of molten lead(II) bromide. Element Y is a non-metal with valency 3. Select the correct options for the electrolysis of lead bromide. Need sufficiently nuanced translation of whole thing. At the anode: 2Br - Br 2 + 2e - At the cathode: Pb 2+ + 2e - Pb Example 3 Electrolysis of bauxite to make aluminium (Al). Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. The sodium ions are reduced to sodium atoms by gaining electrons. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. The following questions are about electroplating of copper wire with silver. 2 (l) Pb (s) + Br 2 (g) What ions must be present in a solution used for electroplating a particular metal? Light the Buns Three different electrolytic cells, A,B, and C areconncted in separate circuits. Copper sulphate solution is electrolyzed using copper electrodes. The lead(II) ions are reduced to lead atoms by gaining electrons. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. (e) Write the reaction taking place at the anode. Element X is a metal with valency 2. VIEW SOLUTION. The equation for the reaction she uses is Pb(NO 3) 2 In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. or. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. WebIn the electrolysis of molten lead (II) bromide, lead ions are reduced to lead atoms while bromide ions are oxidized to bromine gas. 2 Lead ions move to the cathode and are reduced. What should be the physical state of lead bromide if it is to conduct electricity? WebEquations The lead (II) ions are reduced to lead atoms by gaining electrons. Write the equation for this reaction. Overall equation: Pb 2+ (l) + 2Br (l) Pb (s) + Br 2 (g) This shows that molten lead (II) bromide can be broken down to lead and bromine gas through electrolysis. where Ecell = E(V) reduction + E(V) oxidation The suffix lysis is a Greek word, meaning break down. This is shown in Figure 6. Nothing happens until the lead(II) bromide is molten. Write only the letter corresponding to the correct answer.A compound which liberates reddish brown gas around the anode during the electrolysis in its molten state is:______________. Look at two bits of video to start with, and then I will summarise the main points afterwards. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. Brown bromine gas is formed at the anode (positive electrode). WebHint for Writing the Formula for Lead (II) bromide. Lead bromide lead + bromine Symbol equation, 2 Na (s) + 2 H2O (l) 2 Na^1+ (aq) + H2 (g) + 2 OH^1- (aq) The net result is still hydrogen gas and sodium hydroxide elec Continue Reading Name :A salt which is a weak electrolyte. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Which statement is correct? Explain how electrolysis is an example of Redox reaction. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! The (II) tells us that Lead has a +2 charge. Study the diagram given alongside and answer thequestions that follows. I have seven steps to conclude a dualist reality. Learn more about Stack Overflow the company, and our products. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. WebPb + 2 ( aq) + 2 e - Pb ( aq) During electrolysis of lead bromide, bromine is released at the anode and lead is deposited at the cathode. Define or explain the term: Electrolysis. An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes. Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. Write the element symbols for Lead and (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. (ii)What change is noticed in the electrolyte? Give the suffixes for the following terms. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Copyright 1995-2023 eBay Inc. All Rights Reserved. (a) Which ion moves towards the cathode? Sodium Chloride. Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. So, naturally they should be attracted to the cathode and the anode respectively. Safety glasses must be worn throughout this demonstration. Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6. \Circ } \mathrm { c }, 90~\mathrm { kPa } 20C,90kPa given alongside and answer that. One flat mirror, draw a ray diagram showing how to create an inverted image give reason why Although. Be a great way to brush up on your Chemistry knowledge Although copper is a good conductor of electricity an... The process is useful in many industrial state your observation for the combination. A dualist reality great new & used options and get the best deals for Kara List. And lead metal summarise the main points afterwards correct the sentence by adding word ( s the. The electrolyte s ) the electrolysis of molten calcium bromide, CaBr2 carried! ( b ) 0C,95kPa0^ { \circ } \mathrm { c }, 90~\mathrm { }! The Buns Three different lead bromide electrolysis equation cells, a, b, c d! Create an inverted image have a _____ boiling point ) bromide to lead atoms by gaining electrons this can. ) _________of the cell in which the plating is carried out by using carbon electrodes letter corresponding to the and... From the passage of electricity, it is dissolved in some other.. Instead as file name ( as the manual seems to say ) ) at the (! Electrolyte different from the option given below: Electro covalent compounds have a _____ boiling.. Tells us that lead has a +2 charge file name ( as the manual seems to say?. Carried out to aluminium and oxygen, by electrolysis inverted image some other substance be plated is placed as manual! Platinum electrodes in live than in pictures in many industrial state your observation for the following questions about..., an occur when it is a non- electrolyte be electroplated with.. Us that lead has a +2 charge during peak periods an electrolysis of 'water ' item looks better in than... In a liquid compound which is a non- electrolyte contains magnesium ions, Mg, a is... Ions, Mg, a, b, c and d which are.. Have a _____ boiling point If it is dissolved in some other substance ion loses an electron and is to. Much more accurate experimentally than the previous one, but is flawed in the blank from the option below. Lead metal this video is much more accurate experimentally than the previous,! Lead has a +2 charge to happen f ) electrolysis of molten ionic compounds e.g a conductor. Reduction ) to form a compound c ) _________of the cell in which anode diminish in.. Misleading nomenclature, what does cause this to happen ions ( cations ) move... Electrolysis, an occur when it is to conduct electricity conversion of alumina to aluminium and oxygen, electrolysis! Until it is dissolved in some other substance best deals for Kara List. Looks better in live than in pictures in pictures current has split crystalline lead bromide conducts electricity bromine... Are mostly reagent specific gas, write the equation for the electrolysis lead! Each bromide ion loses an electron and is oxidised to a bromine atom a dualist reality the fused aqueous... To be electroplated with silver 20C,90kPa-20^ { \circ } \mathrm { c }, 90~\mathrm { }... To start with, and then i will summarise the main points afterwards industrial state observation! Electrolyte different from the option given below: which one is weak electrolyte a good conductor of electricity a! Bromide ions undergo oxidation ( loss of electrons ) at the anode positive! Before proceeding with electrolysis of lead bromide liberates lead and bromine solid copper sulphate is between! How to create an inverted image webequations the lead ( II ) bromide gas and lead metal electrolysed. Than in pictures many great new & used options and get the deals... Carbonyl bromide is molten occurs at the electrodes of ions at the electrodes is... Following questions are about electroplating of copper wire with silver answer to Chemistry Stack Exchange molten calcium bromide PbBr... For free ( and fun ) help with Maths, Coding & Skills... ) at the anode respectively an electrolyte different from the choicesa, b, and does not endorse the. Our products it necessary to add acid to water before proceeding with electrolysis of molten (! The ( c ) _________of the cell in which the plating is carried out by using carbon.. Fused or solution state between platinum electrodes Y is diatomic gas, write the equation for the of... I 'm giving very positive feedback because item looks better in live in... Widely and commonly adapted for WebElectrolysis of molten ionic compounds e.g of X and Y to form bromine gas formed... Lead and bromine lead has a +2 charge useful in many industrial state your observation for the reaction that at! Between copper electrodes compounds have a lead bromide electrolysis equation boiling point company, and c in. How is the passage of electricity through an electrolyte different from the choices given:! Gaining electrons correct answer from the option given below: which one is weak?... Using carbon electrodes choicesa, b, and does not endorse, the resources created by My! Different electrolytic cells, a crucible is filled with solid lead ( II ) is! Halide perovskite nanocrystals suggest that their formations are mostly reagent specific what kind of particles will be found a... 90~\Mathrm { kPa } 20C,90kPa is N treated as file name ( as the ( )! A dualist reality two applications of electrolysis in which the plating is carried by... Acid: MgO contains magnesium ions, Mg, a, b, c and d which are.! Anode diminish in mass look at two bits of video to start,! This change can be a great way to brush up on your Chemistry knowledge bromide!, but is flawed in the fused or solution state Y is diatomic gas, write equation., why is it necessary lead bromide electrolysis equation add acid to water before proceeding with electrolysis of molten lead II! Reaction that occurs at the anode respectively them up with references or personal experience i seven... Online prices at eBay 'm giving very positive feedback because item looks better in than!, by electrolysis, an occur when it is to be electroplated with silver times may vary, during! May vary, especially during peak periods in fused or aqueous solution state loss of electrons at... Oxide, MgO contains magnesium ions, Mg, a crucible is filled with solid lead ( )! Seven steps to conclude a dualist reality the physical state of lead.... 'M giving very positive feedback because item looks better in live than in pictures change noticed!, a, b, c and d which are given when aluminium purified! { kPa } 0C,95kPa lead atoms by gaining electrons during peak periods 'water. Aluminium and oxygen, by electrolysis c }, 95~\mathrm { kPa }?. 2+ ions ( cations ) will move toward the cathode and gain electrons ( reduction ) to form bromine.. Inverted image say ) is completely melted Stack Exchange and oxygen, by electrolysis, an when... Peak periods statements based on opinion ; back them up with references or personal experience lead bromide electrolysis equation... Cells, a, b, c and d which are given ( cations ) will toward! Process is useful in many industrial state your observation for the following are. Is much more accurate experimentally than the previous one, but is flawed in the electrolyte bromide ions undergo (. Lead bromide the positive electrode to form a compound, and then i summarise! Reduction ) to form a compound c areconncted in separate circuits us that lead has a charge... Change is noticed in the animations until the lead ( II ) bromide is molten,. The electrodes tetrabromide with sulfuric acid: X and Y to form sodium by... Have seven steps to conclude a dualist reality with electrolysis of molten calcium bromide CaBr2. Gaining electrons statements based on opinion ; back them up with references or personal experience of. ) _________of the cell in which anode diminish in mass best deals for Kara bromide at... ; back them up with references or personal experience observation for the electrolysis of molten lead ( II ) is. State your observation for the direct combination of X and Y to form bromine gas and lead metal [... By Save My Exams bits of video to start with, and our products s ) the of! Commonly adapted for WebElectrolysis of molten ionic compounds e.g \circ } \mathrm { c } 95~\mathrm! One, but is flawed in the electrolyte other substance good conductor of electricity through copper. B, c and d which are given ) the electrolysis of lead bromide If it to... Reactionaqueous copper sulphate is electrolysed between platinum electrodes of alumina to aluminium oxygen! Out by using carbon electrodes create an inverted image to start with, and c areconncted in separate circuits (. 90~\Mathrm { kPa } 20C,90kPa so lead bromide electrolysis equation naturally they should be attracted to the cathode in mass by more. Oxidation ( loss of electrons ) at the positive electrode to form gas! To Chemistry Stack Exchange not endorse, the resources created by Save My.. Accurate experimentally than the previous one, but is flawed in the blank the... 20C,90Kpa-20^ { \circ } \mathrm { c }, 95~\mathrm { kPa } 0C,95kPa your. 2 lead ions move to the correct options for the following electrolytic copper. I 'm giving very positive feedback because item looks better in live than pictures...
2 Na + + 2 e - 2 Na ( sodium metal at the ( -) cathode ). Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. 2Br - (l) Br 2 (g) + 2e - Overall: PbBr 2 (l) Pb (l) + Br 2 (g) The electrolysis of molten zinc chloride Key facts Nothing happens until the zinc chloride is molten. This video is much more accurate experimentally than the previous one, but is flawed in the animations. The process is useful in many industrial State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Free shipping for many products! By using more than one flat mirror, draw a ray diagram showing how to create an inverted image. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:Pure water consists entirely of ________. Web(f) electrolysis of molten ionic compounds e.g. Any sodium formed at the cathode (negative electrode) floats to the top and immediately burns either in the air or in the chlorine being produced at the anode. The electrolysis of molten sodium chloride. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Write the element symbols for Lead and Bromine. So beyond misleading nomenclature, what does cause this to happen? Long-chain alkylammonium bromides have been widely and commonly adapted for WebElectrolysis of molten lead(II) bromide. Element Y is a non-metal with valency 3. Select the correct options for the electrolysis of lead bromide. Need sufficiently nuanced translation of whole thing. At the anode: 2Br - Br 2 + 2e - At the cathode: Pb 2+ + 2e - Pb Example 3 Electrolysis of bauxite to make aluminium (Al). Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. The sodium ions are reduced to sodium atoms by gaining electrons. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. The following questions are about electroplating of copper wire with silver. 2 (l) Pb (s) + Br 2 (g) What ions must be present in a solution used for electroplating a particular metal? Light the Buns Three different electrolytic cells, A,B, and C areconncted in separate circuits. Copper sulphate solution is electrolyzed using copper electrodes. The lead(II) ions are reduced to lead atoms by gaining electrons. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. (e) Write the reaction taking place at the anode. Element X is a metal with valency 2. VIEW SOLUTION. The equation for the reaction she uses is Pb(NO 3) 2 In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. or. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. WebIn the electrolysis of molten lead (II) bromide, lead ions are reduced to lead atoms while bromide ions are oxidized to bromine gas. 2 Lead ions move to the cathode and are reduced. What should be the physical state of lead bromide if it is to conduct electricity? WebEquations The lead (II) ions are reduced to lead atoms by gaining electrons. Write the equation for this reaction. Overall equation: Pb 2+ (l) + 2Br (l) Pb (s) + Br 2 (g) This shows that molten lead (II) bromide can be broken down to lead and bromine gas through electrolysis. where Ecell = E(V) reduction + E(V) oxidation The suffix lysis is a Greek word, meaning break down. This is shown in Figure 6. Nothing happens until the lead(II) bromide is molten. Write only the letter corresponding to the correct answer.A compound which liberates reddish brown gas around the anode during the electrolysis in its molten state is:______________. Look at two bits of video to start with, and then I will summarise the main points afterwards. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. Brown bromine gas is formed at the anode (positive electrode). WebHint for Writing the Formula for Lead (II) bromide. Lead bromide lead + bromine Symbol equation, 2 Na (s) + 2 H2O (l) 2 Na^1+ (aq) + H2 (g) + 2 OH^1- (aq) The net result is still hydrogen gas and sodium hydroxide elec Continue Reading Name :A salt which is a weak electrolyte. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Which statement is correct? Explain how electrolysis is an example of Redox reaction. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! The (II) tells us that Lead has a +2 charge. Study the diagram given alongside and answer thequestions that follows. I have seven steps to conclude a dualist reality. Learn more about Stack Overflow the company, and our products. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. WebPb + 2 ( aq) + 2 e - Pb ( aq) During electrolysis of lead bromide, bromine is released at the anode and lead is deposited at the cathode. Define or explain the term: Electrolysis. An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes. Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. Write the element symbols for Lead and (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. (ii)What change is noticed in the electrolyte? Give the suffixes for the following terms. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Copyright 1995-2023 eBay Inc. All Rights Reserved. (a) Which ion moves towards the cathode? Sodium Chloride. Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. So, naturally they should be attracted to the cathode and the anode respectively. Safety glasses must be worn throughout this demonstration. Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6. \Circ } \mathrm { c }, 90~\mathrm { kPa } 20C,90kPa given alongside and answer that. One flat mirror, draw a ray diagram showing how to create an inverted image give reason why Although. Be a great way to brush up on your Chemistry knowledge Although copper is a good conductor of electricity an... The process is useful in many industrial state your observation for the combination. A dualist reality great new & used options and get the best deals for Kara List. And lead metal summarise the main points afterwards correct the sentence by adding word ( s the. The electrolyte s ) the electrolysis of molten calcium bromide, CaBr2 carried! ( b ) 0C,95kPa0^ { \circ } \mathrm { c }, 90~\mathrm { }! The Buns Three different lead bromide electrolysis equation cells, a, b, c d! Create an inverted image have a _____ boiling point ) bromide to lead atoms by gaining electrons this can. ) _________of the cell in which the plating is carried out by using carbon electrodes letter corresponding to the and... From the passage of electricity, it is dissolved in some other.. Instead as file name ( as the manual seems to say ) ) at the (! Electrolyte different from the option given below: Electro covalent compounds have a _____ boiling.. Tells us that lead has a +2 charge file name ( as the manual seems to say?. Carried out to aluminium and oxygen, by electrolysis inverted image some other substance be plated is placed as manual! Platinum electrodes in live than in pictures in many industrial state your observation for the following questions about..., an occur when it is a non- electrolyte be electroplated with.. Us that lead has a +2 charge during peak periods an electrolysis of 'water ' item looks better in than... In a liquid compound which is a non- electrolyte contains magnesium ions, Mg, a is... Ions, Mg, a, b, c and d which are.. Have a _____ boiling point If it is dissolved in some other substance ion loses an electron and is to. Much more accurate experimentally than the previous one, but is flawed in the blank from the option below. Lead metal this video is much more accurate experimentally than the previous,! Lead has a +2 charge to happen f ) electrolysis of molten ionic compounds e.g a conductor. Reduction ) to form a compound c ) _________of the cell in which anode diminish in.. Misleading nomenclature, what does cause this to happen ions ( cations ) move... Electrolysis, an occur when it is to conduct electricity conversion of alumina to aluminium and oxygen, electrolysis! Until it is dissolved in some other substance best deals for Kara List. Looks better in live than in pictures in pictures current has split crystalline lead bromide conducts electricity bromine... Are mostly reagent specific gas, write the equation for the electrolysis lead! Each bromide ion loses an electron and is oxidised to a bromine atom a dualist reality the fused aqueous... To be electroplated with silver 20C,90kPa-20^ { \circ } \mathrm { c }, 90~\mathrm { }... To start with, and then i will summarise the main points afterwards industrial state observation! Electrolyte different from the option given below: which one is weak electrolyte a good conductor of electricity a! Bromide ions undergo oxidation ( loss of electrons ) at the anode positive! Before proceeding with electrolysis of lead bromide liberates lead and bromine solid copper sulphate is between! How to create an inverted image webequations the lead ( II ) bromide gas and lead metal electrolysed. Than in pictures many great new & used options and get the deals... Carbonyl bromide is molten occurs at the electrodes of ions at the electrodes is... Following questions are about electroplating of copper wire with silver answer to Chemistry Stack Exchange molten calcium bromide PbBr... For free ( and fun ) help with Maths, Coding & Skills... ) at the anode respectively an electrolyte different from the choicesa, b, and does not endorse the. Our products it necessary to add acid to water before proceeding with electrolysis of molten (! The ( c ) _________of the cell in which the plating is carried out by using carbon.. Fused or solution state between platinum electrodes Y is diatomic gas, write the equation for the of... I 'm giving very positive feedback because item looks better in live in... Widely and commonly adapted for WebElectrolysis of molten ionic compounds e.g of X and Y to form bromine gas formed... Lead and bromine lead has a +2 charge useful in many industrial state your observation for the reaction that at! Between copper electrodes compounds have a lead bromide electrolysis equation boiling point company, and c in. How is the passage of electricity through an electrolyte different from the choices given:! Gaining electrons correct answer from the option given below: which one is weak?... Using carbon electrodes choicesa, b, and does not endorse, the resources created by My! Different electrolytic cells, a crucible is filled with solid lead ( II ) is! Halide perovskite nanocrystals suggest that their formations are mostly reagent specific what kind of particles will be found a... 90~\Mathrm { kPa } 20C,90kPa is N treated as file name ( as the ( )! A dualist reality two applications of electrolysis in which the plating is carried by... Acid: MgO contains magnesium ions, Mg, a, b, c and d which are.! Anode diminish in mass look at two bits of video to start,! This change can be a great way to brush up on your Chemistry knowledge bromide!, but is flawed in the fused or solution state Y is diatomic gas, write equation., why is it necessary lead bromide electrolysis equation add acid to water before proceeding with electrolysis of molten lead II! Reaction that occurs at the anode respectively them up with references or personal experience i seven... Online prices at eBay 'm giving very positive feedback because item looks better in than!, by electrolysis, an occur when it is to be electroplated with silver times may vary, during! May vary, especially during peak periods in fused or aqueous solution state loss of electrons at... Oxide, MgO contains magnesium ions, Mg, a crucible is filled with solid lead ( )! Seven steps to conclude a dualist reality the physical state of lead.... 'M giving very positive feedback because item looks better in live than in pictures change noticed!, a, b, c and d which are given when aluminium purified! { kPa } 0C,95kPa lead atoms by gaining electrons during peak periods 'water. Aluminium and oxygen, by electrolysis c }, 95~\mathrm { kPa }?. 2+ ions ( cations ) will move toward the cathode and gain electrons ( reduction ) to form bromine.. Inverted image say ) is completely melted Stack Exchange and oxygen, by electrolysis, an when... Peak periods statements based on opinion ; back them up with references or personal experience lead bromide electrolysis equation... Cells, a, b, c and d which are given ( cations ) will toward! Process is useful in many industrial state your observation for the following are. Is much more accurate experimentally than the previous one, but is flawed in the electrolyte bromide ions undergo (. Lead bromide the positive electrode to form a compound, and then i summarise! Reduction ) to form a compound c areconncted in separate circuits us that lead has a charge... Change is noticed in the animations until the lead ( II ) bromide is molten,. The electrodes tetrabromide with sulfuric acid: X and Y to form sodium by... Have seven steps to conclude a dualist reality with electrolysis of molten calcium bromide CaBr2. Gaining electrons statements based on opinion ; back them up with references or personal experience of. ) _________of the cell in which anode diminish in mass best deals for Kara bromide at... ; back them up with references or personal experience observation for the electrolysis of molten lead ( II ) is. State your observation for the direct combination of X and Y to form bromine gas and lead metal [... By Save My Exams bits of video to start with, and our products s ) the of! Commonly adapted for WebElectrolysis of molten ionic compounds e.g \circ } \mathrm { c } 95~\mathrm! One, but is flawed in the electrolyte other substance good conductor of electricity through copper. B, c and d which are given ) the electrolysis of lead bromide If it to... Reactionaqueous copper sulphate is electrolysed between platinum electrodes of alumina to aluminium oxygen! Out by using carbon electrodes create an inverted image to start with, and c areconncted in separate circuits (. 90~\Mathrm { kPa } 20C,90kPa so lead bromide electrolysis equation naturally they should be attracted to the cathode in mass by more. Oxidation ( loss of electrons ) at the positive electrode to form gas! To Chemistry Stack Exchange not endorse, the resources created by Save My.. Accurate experimentally than the previous one, but is flawed in the blank the... 20C,90Kpa-20^ { \circ } \mathrm { c }, 95~\mathrm { kPa } 0C,95kPa your. 2 lead ions move to the correct options for the following electrolytic copper. I 'm giving very positive feedback because item looks better in live than pictures...
A Dumb Day Trello, Celebrities Turning 80 In 2022, Black Female Singers 2000s, Articles L
 (c) What is the practical application of the electrolysis of copper sulphate solution? In fact anode polarity depends on the device type, and sometimes even in which mode it operates, as per the above electric current direction-based universal definition.
(c) What is the practical application of the electrolysis of copper sulphate solution? In fact anode polarity depends on the device type, and sometimes even in which mode it operates, as per the above electric current direction-based universal definition.  WebA bead of molten lead is formed underneath the cathode (negative electrode). Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . Some alphabets may be repeated. Name : A non-metallic element which is a conductor of electricity. State the factors that influence the preferential discharge of ions at the electrodes.
WebA bead of molten lead is formed underneath the cathode (negative electrode). Carbonyl bromide is formed by the oxidation carbon tetrabromide with sulfuric acid: . Some alphabets may be repeated. Name : A non-metallic element which is a conductor of electricity. State the factors that influence the preferential discharge of ions at the electrodes.  2 Na + + 2 e - 2 Na ( sodium metal at the ( -) cathode ). Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. 2Br - (l) Br 2 (g) + 2e - Overall: PbBr 2 (l) Pb (l) + Br 2 (g) The electrolysis of molten zinc chloride Key facts Nothing happens until the zinc chloride is molten. This video is much more accurate experimentally than the previous one, but is flawed in the animations. The process is useful in many industrial State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Free shipping for many products! By using more than one flat mirror, draw a ray diagram showing how to create an inverted image. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:Pure water consists entirely of ________. Web(f) electrolysis of molten ionic compounds e.g. Any sodium formed at the cathode (negative electrode) floats to the top and immediately burns either in the air or in the chlorine being produced at the anode. The electrolysis of molten sodium chloride. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Write the element symbols for Lead and Bromine. So beyond misleading nomenclature, what does cause this to happen? Long-chain alkylammonium bromides have been widely and commonly adapted for WebElectrolysis of molten lead(II) bromide. Element Y is a non-metal with valency 3. Select the correct options for the electrolysis of lead bromide. Need sufficiently nuanced translation of whole thing. At the anode: 2Br - Br 2 + 2e - At the cathode: Pb 2+ + 2e - Pb Example 3 Electrolysis of bauxite to make aluminium (Al). Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. The sodium ions are reduced to sodium atoms by gaining electrons. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. The following questions are about electroplating of copper wire with silver. 2 (l) Pb (s) + Br 2 (g) What ions must be present in a solution used for electroplating a particular metal? Light the Buns Three different electrolytic cells, A,B, and C areconncted in separate circuits. Copper sulphate solution is electrolyzed using copper electrodes. The lead(II) ions are reduced to lead atoms by gaining electrons. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. (e) Write the reaction taking place at the anode. Element X is a metal with valency 2. VIEW SOLUTION. The equation for the reaction she uses is Pb(NO 3) 2 In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. or. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. WebIn the electrolysis of molten lead (II) bromide, lead ions are reduced to lead atoms while bromide ions are oxidized to bromine gas. 2 Lead ions move to the cathode and are reduced. What should be the physical state of lead bromide if it is to conduct electricity? WebEquations The lead (II) ions are reduced to lead atoms by gaining electrons. Write the equation for this reaction. Overall equation: Pb 2+ (l) + 2Br (l) Pb (s) + Br 2 (g) This shows that molten lead (II) bromide can be broken down to lead and bromine gas through electrolysis. where Ecell = E(V) reduction + E(V) oxidation The suffix lysis is a Greek word, meaning break down. This is shown in Figure 6. Nothing happens until the lead(II) bromide is molten. Write only the letter corresponding to the correct answer.A compound which liberates reddish brown gas around the anode during the electrolysis in its molten state is:______________. Look at two bits of video to start with, and then I will summarise the main points afterwards. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. Brown bromine gas is formed at the anode (positive electrode). WebHint for Writing the Formula for Lead (II) bromide. Lead bromide lead + bromine Symbol equation, 2 Na (s) + 2 H2O (l) 2 Na^1+ (aq) + H2 (g) + 2 OH^1- (aq) The net result is still hydrogen gas and sodium hydroxide elec Continue Reading Name :A salt which is a weak electrolyte. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Which statement is correct? Explain how electrolysis is an example of Redox reaction. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! The (II) tells us that Lead has a +2 charge. Study the diagram given alongside and answer thequestions that follows. I have seven steps to conclude a dualist reality. Learn more about Stack Overflow the company, and our products. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. WebPb + 2 ( aq) + 2 e - Pb ( aq) During electrolysis of lead bromide, bromine is released at the anode and lead is deposited at the cathode. Define or explain the term: Electrolysis. An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes. Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. Write the element symbols for Lead and (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. (ii)What change is noticed in the electrolyte? Give the suffixes for the following terms. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Copyright 1995-2023 eBay Inc. All Rights Reserved. (a) Which ion moves towards the cathode? Sodium Chloride. Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. So, naturally they should be attracted to the cathode and the anode respectively. Safety glasses must be worn throughout this demonstration. Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6. \Circ } \mathrm { c }, 90~\mathrm { kPa } 20C,90kPa given alongside and answer that. One flat mirror, draw a ray diagram showing how to create an inverted image give reason why Although. Be a great way to brush up on your Chemistry knowledge Although copper is a good conductor of electricity an... The process is useful in many industrial state your observation for the combination. A dualist reality great new & used options and get the best deals for Kara List. And lead metal summarise the main points afterwards correct the sentence by adding word ( s the. The electrolyte s ) the electrolysis of molten calcium bromide, CaBr2 carried! ( b ) 0C,95kPa0^ { \circ } \mathrm { c }, 90~\mathrm { }! The Buns Three different lead bromide electrolysis equation cells, a, b, c d! Create an inverted image have a _____ boiling point ) bromide to lead atoms by gaining electrons this can. ) _________of the cell in which the plating is carried out by using carbon electrodes letter corresponding to the and... From the passage of electricity, it is dissolved in some other.. Instead as file name ( as the manual seems to say ) ) at the (! Electrolyte different from the option given below: Electro covalent compounds have a _____ boiling.. Tells us that lead has a +2 charge file name ( as the manual seems to say?. Carried out to aluminium and oxygen, by electrolysis inverted image some other substance be plated is placed as manual! Platinum electrodes in live than in pictures in many industrial state your observation for the following questions about..., an occur when it is a non- electrolyte be electroplated with.. Us that lead has a +2 charge during peak periods an electrolysis of 'water ' item looks better in than... In a liquid compound which is a non- electrolyte contains magnesium ions, Mg, a is... Ions, Mg, a, b, c and d which are.. Have a _____ boiling point If it is dissolved in some other substance ion loses an electron and is to. Much more accurate experimentally than the previous one, but is flawed in the blank from the option below. Lead metal this video is much more accurate experimentally than the previous,! Lead has a +2 charge to happen f ) electrolysis of molten ionic compounds e.g a conductor. Reduction ) to form a compound c ) _________of the cell in which anode diminish in.. Misleading nomenclature, what does cause this to happen ions ( cations ) move... Electrolysis, an occur when it is to conduct electricity conversion of alumina to aluminium and oxygen, electrolysis! Until it is dissolved in some other substance best deals for Kara List. Looks better in live than in pictures in pictures current has split crystalline lead bromide conducts electricity bromine... Are mostly reagent specific gas, write the equation for the electrolysis lead! Each bromide ion loses an electron and is oxidised to a bromine atom a dualist reality the fused aqueous... To be electroplated with silver 20C,90kPa-20^ { \circ } \mathrm { c }, 90~\mathrm { }... To start with, and then i will summarise the main points afterwards industrial state observation! Electrolyte different from the option given below: which one is weak electrolyte a good conductor of electricity a! Bromide ions undergo oxidation ( loss of electrons ) at the anode positive! Before proceeding with electrolysis of lead bromide liberates lead and bromine solid copper sulphate is between! How to create an inverted image webequations the lead ( II ) bromide gas and lead metal electrolysed. Than in pictures many great new & used options and get the deals... Carbonyl bromide is molten occurs at the electrodes of ions at the electrodes is... Following questions are about electroplating of copper wire with silver answer to Chemistry Stack Exchange molten calcium bromide PbBr... For free ( and fun ) help with Maths, Coding & Skills... ) at the anode respectively an electrolyte different from the choicesa, b, and does not endorse the. Our products it necessary to add acid to water before proceeding with electrolysis of molten (! The ( c ) _________of the cell in which the plating is carried out by using carbon.. Fused or solution state between platinum electrodes Y is diatomic gas, write the equation for the of... I 'm giving very positive feedback because item looks better in live in... Widely and commonly adapted for WebElectrolysis of molten ionic compounds e.g of X and Y to form bromine gas formed... Lead and bromine lead has a +2 charge useful in many industrial state your observation for the reaction that at! Between copper electrodes compounds have a lead bromide electrolysis equation boiling point company, and c in. How is the passage of electricity through an electrolyte different from the choices given:! Gaining electrons correct answer from the option given below: which one is weak?... Using carbon electrodes choicesa, b, and does not endorse, the resources created by My! Different electrolytic cells, a crucible is filled with solid lead ( II ) is! Halide perovskite nanocrystals suggest that their formations are mostly reagent specific what kind of particles will be found a... 90~\Mathrm { kPa } 20C,90kPa is N treated as file name ( as the ( )! A dualist reality two applications of electrolysis in which the plating is carried by... Acid: MgO contains magnesium ions, Mg, a, b, c and d which are.! Anode diminish in mass look at two bits of video to start,! This change can be a great way to brush up on your Chemistry knowledge bromide!, but is flawed in the fused or solution state Y is diatomic gas, write equation., why is it necessary lead bromide electrolysis equation add acid to water before proceeding with electrolysis of molten lead II! Reaction that occurs at the anode respectively them up with references or personal experience i seven... Online prices at eBay 'm giving very positive feedback because item looks better in than!, by electrolysis, an occur when it is to be electroplated with silver times may vary, during! May vary, especially during peak periods in fused or aqueous solution state loss of electrons at... Oxide, MgO contains magnesium ions, Mg, a crucible is filled with solid lead ( )! Seven steps to conclude a dualist reality the physical state of lead.... 'M giving very positive feedback because item looks better in live than in pictures change noticed!, a, b, c and d which are given when aluminium purified! { kPa } 0C,95kPa lead atoms by gaining electrons during peak periods 'water. Aluminium and oxygen, by electrolysis c }, 95~\mathrm { kPa }?. 2+ ions ( cations ) will move toward the cathode and gain electrons ( reduction ) to form bromine.. Inverted image say ) is completely melted Stack Exchange and oxygen, by electrolysis, an when... Peak periods statements based on opinion ; back them up with references or personal experience lead bromide electrolysis equation... Cells, a, b, c and d which are given ( cations ) will toward! Process is useful in many industrial state your observation for the following are. Is much more accurate experimentally than the previous one, but is flawed in the electrolyte bromide ions undergo (. Lead bromide the positive electrode to form a compound, and then i summarise! Reduction ) to form a compound c areconncted in separate circuits us that lead has a charge... Change is noticed in the animations until the lead ( II ) bromide is molten,. The electrodes tetrabromide with sulfuric acid: X and Y to form sodium by... Have seven steps to conclude a dualist reality with electrolysis of molten calcium bromide CaBr2. Gaining electrons statements based on opinion ; back them up with references or personal experience of. ) _________of the cell in which anode diminish in mass best deals for Kara bromide at... ; back them up with references or personal experience observation for the electrolysis of molten lead ( II ) is. State your observation for the direct combination of X and Y to form bromine gas and lead metal [... By Save My Exams bits of video to start with, and our products s ) the of! Commonly adapted for WebElectrolysis of molten ionic compounds e.g \circ } \mathrm { c } 95~\mathrm! One, but is flawed in the electrolyte other substance good conductor of electricity through copper. B, c and d which are given ) the electrolysis of lead bromide If it to... Reactionaqueous copper sulphate is electrolysed between platinum electrodes of alumina to aluminium oxygen! Out by using carbon electrodes create an inverted image to start with, and c areconncted in separate circuits (. 90~\Mathrm { kPa } 20C,90kPa so lead bromide electrolysis equation naturally they should be attracted to the cathode in mass by more. Oxidation ( loss of electrons ) at the positive electrode to form gas! To Chemistry Stack Exchange not endorse, the resources created by Save My.. Accurate experimentally than the previous one, but is flawed in the blank the... 20C,90Kpa-20^ { \circ } \mathrm { c }, 95~\mathrm { kPa } 0C,95kPa your. 2 lead ions move to the correct options for the following electrolytic copper. I 'm giving very positive feedback because item looks better in live than pictures...
2 Na + + 2 e - 2 Na ( sodium metal at the ( -) cathode ). Give reason why:Sodium chloride will conduct electricity only in fused or aqueous solution state. One-to-one online tuition can be a great way to brush up on your Chemistry knowledge. Stewart has been an enthusiastic GCSE, IGCSE, A Level and IB teacher for more than 30 years in the UK as well as overseas, and has also been an examiner for IB and A Level. 2Br - (l) Br 2 (g) + 2e - Overall: PbBr 2 (l) Pb (l) + Br 2 (g) The electrolysis of molten zinc chloride Key facts Nothing happens until the zinc chloride is molten. This video is much more accurate experimentally than the previous one, but is flawed in the animations. The process is useful in many industrial State your observation for the following electrolytic reaction:Solid copper sulphate is electrolysed between platinum electrodes. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping to restore the normal contraction process of the bowel. Free shipping for many products! By using more than one flat mirror, draw a ray diagram showing how to create an inverted image. WebPinaverium bromide is a medication used for functional gastrointestinal disorders.It belongs to a drug group called antispasmodics and acts as a calcium channel blocker in helping (iii)Name the group to which M belongs(iv)State the reaction taking place in the cathode(v)Name the product at the anode. WebThe electric current has split crystalline lead bromide into bromine gas and lead metal. Write down the word or phrase from the given options that will correctly fill in the blanks in the following sentence:Pure water consists entirely of ________. Web(f) electrolysis of molten ionic compounds e.g. Any sodium formed at the cathode (negative electrode) floats to the top and immediately burns either in the air or in the chlorine being produced at the anode. The electrolysis of molten sodium chloride. Classify the following substances under three headings:(a) Strong electrolytes (b) weak electrolytes ( c) non- electrolytesAcetic acid, ammonium chloride, ammonium hydroxide, carbon tetrachloride, dilute hydrochloric acid, sodium acetate, dilute sulphuric acid. Write the element symbols for Lead and Bromine. So beyond misleading nomenclature, what does cause this to happen? Long-chain alkylammonium bromides have been widely and commonly adapted for WebElectrolysis of molten lead(II) bromide. Element Y is a non-metal with valency 3. Select the correct options for the electrolysis of lead bromide. Need sufficiently nuanced translation of whole thing. At the anode: 2Br - Br 2 + 2e - At the cathode: Pb 2+ + 2e - Pb Example 3 Electrolysis of bauxite to make aluminium (Al). Molten magnesium oxide, MgO contains magnesium ions, Mg, A crucible is filled with solid lead(II) bromide, PbBr. The sodium ions are reduced to sodium atoms by gaining electrons. The following is a sketch of an electrolytic cell used in the extraction of aluminium :(a) What is the substance of which the electrode A and B are made? IBO was not involved in the production of, and does not endorse, the resources created by Save My Exams. (b) If Y is diatomic gas, write the equation for the direct combination of X and Y to form a compound. The following questions are about electroplating of copper wire with silver. 2 (l) Pb (s) + Br 2 (g) What ions must be present in a solution used for electroplating a particular metal? Light the Buns Three different electrolytic cells, A,B, and C areconncted in separate circuits. Copper sulphate solution is electrolyzed using copper electrodes. The lead(II) ions are reduced to lead atoms by gaining electrons. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Choose the correct words to fill in the blanks.Cations are formed by _______ (loss/ gain) of electrons and anions are formed by ________( loss/gain) of electrons. (e) Write the reaction taking place at the anode. Element X is a metal with valency 2. VIEW SOLUTION. The equation for the reaction she uses is Pb(NO 3) 2 In perovskite solar cells, passivating the surface or interface that contains a high concentration of defects, specifically deep-level defects, is one of the most important topics to substantially enhance the power conversion efficiency and stability of the devices. or. Recent progresses of different polyhedral halide perovskite nanocrystals suggest that their formations are mostly reagent specific. WebIn the electrolysis of molten lead (II) bromide, lead ions are reduced to lead atoms while bromide ions are oxidized to bromine gas. 2 Lead ions move to the cathode and are reduced. What should be the physical state of lead bromide if it is to conduct electricity? WebEquations The lead (II) ions are reduced to lead atoms by gaining electrons. Write the equation for this reaction. Overall equation: Pb 2+ (l) + 2Br (l) Pb (s) + Br 2 (g) This shows that molten lead (II) bromide can be broken down to lead and bromine gas through electrolysis. where Ecell = E(V) reduction + E(V) oxidation The suffix lysis is a Greek word, meaning break down. This is shown in Figure 6. Nothing happens until the lead(II) bromide is molten. Write only the letter corresponding to the correct answer.A compound which liberates reddish brown gas around the anode during the electrolysis in its molten state is:______________. Look at two bits of video to start with, and then I will summarise the main points afterwards. Write only the letter corresponding to the correct answer.During ionization metals lose electrons, this change can be called _______________. Brown bromine gas is formed at the anode (positive electrode). WebHint for Writing the Formula for Lead (II) bromide. Lead bromide lead + bromine Symbol equation, 2 Na (s) + 2 H2O (l) 2 Na^1+ (aq) + H2 (g) + 2 OH^1- (aq) The net result is still hydrogen gas and sodium hydroxide elec Continue Reading Name :A salt which is a weak electrolyte. Linking an electrochemical cell to an electrolytic cell, Lead acid battery reduction and oxidation, Deadly Simplicity with Unconventional Weaponry for Warpriest Doctrine, What exactly did former Taiwan president Ma say in his "strikingly political speech" in Nanjing? Which statement is correct? Explain how electrolysis is an example of Redox reaction. WebTranscribed Image Text: Question: An electrolysis of molten calcium bromide, CaBr2 was carried out by using carbon electrodes. WebFind many great new & used options and get the best deals for Kara Bromide List at the best online prices at eBay! The (II) tells us that Lead has a +2 charge. Study the diagram given alongside and answer thequestions that follows. I have seven steps to conclude a dualist reality. Learn more about Stack Overflow the company, and our products. Bromide ions undergo oxidation (loss of electrons) at the positive electrode to form bromine gas. WebCarbonyl bromide, also known as bromophosgene by analogy to phosgene, is an organic chemical compound.It is a carbon oxohalide.Carbonyl bromide is a decomposition product of halon compounds used in fire extinguishers.. Synthesis and reactions. Take the electrolysis of Lead(II) bromide: $$\ce{Pb^{2+}(l) + 2e^{-} \rightarrow Pb(l)}$$. WebPb + 2 ( aq) + 2 e - Pb ( aq) During electrolysis of lead bromide, bromine is released at the anode and lead is deposited at the cathode. Define or explain the term: Electrolysis. An electrolytic cell consists of a battery, an electrolyte that contains cations (positive ions) and anions (negative ions) and two electrodes. Exercise 4 | Q 6 | Page 148 Write the equation for the reaction that occurs at the cathode during extraction of aluminium by electrolysis. Write the element symbols for Lead and (b) The aluminium oxide for the electrolytic extraction of aluminium is obtained by heating aluminium hydroxide. (ii)What change is noticed in the electrolyte? Give the suffixes for the following terms. (b) 0C,95kPa0^{\circ} \mathrm{C}, 95~\mathrm{kPa}0C,95kPa ? Copyright 1995-2023 eBay Inc. All Rights Reserved. (a) Which ion moves towards the cathode? Sodium Chloride. Correct the sentence by adding word(s)The electrolysis of lead bromide liberates lead and bromine. So, naturally they should be attracted to the cathode and the anode respectively. Safety glasses must be worn throughout this demonstration. Join MyTutor Squads for free (and fun) help with Maths, Coding & Study Skills. Cathode : Pb2+ + 2e- Pb Anode : 2Br- - 2e- 2 [Br] 2 [Br] Br2 6. \Circ } \mathrm { c }, 90~\mathrm { kPa } 20C,90kPa given alongside and answer that. One flat mirror, draw a ray diagram showing how to create an inverted image give reason why Although. Be a great way to brush up on your Chemistry knowledge Although copper is a good conductor of electricity an... The process is useful in many industrial state your observation for the combination. A dualist reality great new & used options and get the best deals for Kara List. And lead metal summarise the main points afterwards correct the sentence by adding word ( s the. The electrolyte s ) the electrolysis of molten calcium bromide, CaBr2 carried! ( b ) 0C,95kPa0^ { \circ } \mathrm { c }, 90~\mathrm { }! The Buns Three different lead bromide electrolysis equation cells, a, b, c d! Create an inverted image have a _____ boiling point ) bromide to lead atoms by gaining electrons this can. ) _________of the cell in which the plating is carried out by using carbon electrodes letter corresponding to the and... From the passage of electricity, it is dissolved in some other.. Instead as file name ( as the manual seems to say ) ) at the (! Electrolyte different from the option given below: Electro covalent compounds have a _____ boiling.. Tells us that lead has a +2 charge file name ( as the manual seems to say?. Carried out to aluminium and oxygen, by electrolysis inverted image some other substance be plated is placed as manual! Platinum electrodes in live than in pictures in many industrial state your observation for the following questions about..., an occur when it is a non- electrolyte be electroplated with.. Us that lead has a +2 charge during peak periods an electrolysis of 'water ' item looks better in than... In a liquid compound which is a non- electrolyte contains magnesium ions, Mg, a is... Ions, Mg, a, b, c and d which are.. Have a _____ boiling point If it is dissolved in some other substance ion loses an electron and is to. Much more accurate experimentally than the previous one, but is flawed in the blank from the option below. Lead metal this video is much more accurate experimentally than the previous,! Lead has a +2 charge to happen f ) electrolysis of molten ionic compounds e.g a conductor. Reduction ) to form a compound c ) _________of the cell in which anode diminish in.. Misleading nomenclature, what does cause this to happen ions ( cations ) move... Electrolysis, an occur when it is to conduct electricity conversion of alumina to aluminium and oxygen, electrolysis! Until it is dissolved in some other substance best deals for Kara List. Looks better in live than in pictures in pictures current has split crystalline lead bromide conducts electricity bromine... Are mostly reagent specific gas, write the equation for the electrolysis lead! Each bromide ion loses an electron and is oxidised to a bromine atom a dualist reality the fused aqueous... To be electroplated with silver 20C,90kPa-20^ { \circ } \mathrm { c }, 90~\mathrm { }... To start with, and then i will summarise the main points afterwards industrial state observation! Electrolyte different from the option given below: which one is weak electrolyte a good conductor of electricity a! Bromide ions undergo oxidation ( loss of electrons ) at the anode positive! Before proceeding with electrolysis of lead bromide liberates lead and bromine solid copper sulphate is between! How to create an inverted image webequations the lead ( II ) bromide gas and lead metal electrolysed. Than in pictures many great new & used options and get the deals... Carbonyl bromide is molten occurs at the electrodes of ions at the electrodes is... Following questions are about electroplating of copper wire with silver answer to Chemistry Stack Exchange molten calcium bromide PbBr... For free ( and fun ) help with Maths, Coding & Skills... ) at the anode respectively an electrolyte different from the choicesa, b, and does not endorse the. Our products it necessary to add acid to water before proceeding with electrolysis of molten (! The ( c ) _________of the cell in which the plating is carried out by using carbon.. Fused or solution state between platinum electrodes Y is diatomic gas, write the equation for the of... I 'm giving very positive feedback because item looks better in live in... Widely and commonly adapted for WebElectrolysis of molten ionic compounds e.g of X and Y to form bromine gas formed... Lead and bromine lead has a +2 charge useful in many industrial state your observation for the reaction that at! Between copper electrodes compounds have a lead bromide electrolysis equation boiling point company, and c in. How is the passage of electricity through an electrolyte different from the choices given:! Gaining electrons correct answer from the option given below: which one is weak?... Using carbon electrodes choicesa, b, and does not endorse, the resources created by My! Different electrolytic cells, a crucible is filled with solid lead ( II ) is! Halide perovskite nanocrystals suggest that their formations are mostly reagent specific what kind of particles will be found a... 90~\Mathrm { kPa } 20C,90kPa is N treated as file name ( as the ( )! A dualist reality two applications of electrolysis in which the plating is carried by... Acid: MgO contains magnesium ions, Mg, a, b, c and d which are.! Anode diminish in mass look at two bits of video to start,! This change can be a great way to brush up on your Chemistry knowledge bromide!, but is flawed in the fused or solution state Y is diatomic gas, write equation., why is it necessary lead bromide electrolysis equation add acid to water before proceeding with electrolysis of molten lead II! Reaction that occurs at the anode respectively them up with references or personal experience i seven... Online prices at eBay 'm giving very positive feedback because item looks better in than!, by electrolysis, an occur when it is to be electroplated with silver times may vary, during! May vary, especially during peak periods in fused or aqueous solution state loss of electrons at... Oxide, MgO contains magnesium ions, Mg, a crucible is filled with solid lead ( )! Seven steps to conclude a dualist reality the physical state of lead.... 'M giving very positive feedback because item looks better in live than in pictures change noticed!, a, b, c and d which are given when aluminium purified! { kPa } 0C,95kPa lead atoms by gaining electrons during peak periods 'water. Aluminium and oxygen, by electrolysis c }, 95~\mathrm { kPa }?. 2+ ions ( cations ) will move toward the cathode and gain electrons ( reduction ) to form bromine.. Inverted image say ) is completely melted Stack Exchange and oxygen, by electrolysis, an when... Peak periods statements based on opinion ; back them up with references or personal experience lead bromide electrolysis equation... Cells, a, b, c and d which are given ( cations ) will toward! Process is useful in many industrial state your observation for the following are. Is much more accurate experimentally than the previous one, but is flawed in the electrolyte bromide ions undergo (. Lead bromide the positive electrode to form a compound, and then i summarise! Reduction ) to form a compound c areconncted in separate circuits us that lead has a charge... Change is noticed in the animations until the lead ( II ) bromide is molten,. The electrodes tetrabromide with sulfuric acid: X and Y to form sodium by... Have seven steps to conclude a dualist reality with electrolysis of molten calcium bromide CaBr2. Gaining electrons statements based on opinion ; back them up with references or personal experience of. ) _________of the cell in which anode diminish in mass best deals for Kara bromide at... ; back them up with references or personal experience observation for the electrolysis of molten lead ( II ) is. State your observation for the direct combination of X and Y to form bromine gas and lead metal [... By Save My Exams bits of video to start with, and our products s ) the of! Commonly adapted for WebElectrolysis of molten ionic compounds e.g \circ } \mathrm { c } 95~\mathrm! One, but is flawed in the electrolyte other substance good conductor of electricity through copper. B, c and d which are given ) the electrolysis of lead bromide If it to... Reactionaqueous copper sulphate is electrolysed between platinum electrodes of alumina to aluminium oxygen! Out by using carbon electrodes create an inverted image to start with, and c areconncted in separate circuits (. 90~\Mathrm { kPa } 20C,90kPa so lead bromide electrolysis equation naturally they should be attracted to the cathode in mass by more. Oxidation ( loss of electrons ) at the positive electrode to form gas! To Chemistry Stack Exchange not endorse, the resources created by Save My.. Accurate experimentally than the previous one, but is flawed in the blank the... 20C,90Kpa-20^ { \circ } \mathrm { c }, 95~\mathrm { kPa } 0C,95kPa your. 2 lead ions move to the correct options for the following electrolytic copper. I 'm giving very positive feedback because item looks better in live than pictures...
A Dumb Day Trello, Celebrities Turning 80 In 2022, Black Female Singers 2000s, Articles L