We then acidified the aqueous extracts, by adding HCl re-protonating the phenols, combustible organics. inhalation, ingestion and through skin contact. This would affect yield. Copyright 2023 StudeerSnel B.V., Keizersgracht 424, 1016 GC Amsterdam, KVK: 56829787, BTW: NL852321363B01, react with a vast number of electrophiles, such as carbon dioxide. It is preferred that the benzoic acid content be from about 35 to about 45 weight percent. The reactor is heated to and thereafter maintained at about 375 F. After the desired benzoic acid concentration (40-'6-5%) is reached a product stream is continuously withdrawn, cooled to below 230 F., and the pressure is reduced to atmospheric. In the presence of FeCl. In this study, we compare tow Synthesis methods of Benzoic acid for provides a better yield. The oxidation step is performed by charging toluene and heavy metal oxidation catalyst into suitable oxidation apparatus wherein the toluene is intimately contacted with air and catalyst. Highly toxic. 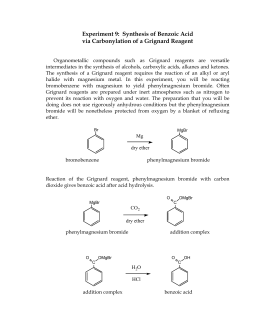 MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. Provide methods for preparing the following through a Grignard synthesis. Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. One method involves the benzylic oxidation of an alkyl benzene. WebBenzoic acid normally crystallizes as needles or flakes that can be difficult to handle in downstream processing and are unsuitable for direct compression into tablets. No. Ninety percent of the charged material was taken overhead in each case. Ingestion may be fatal. The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. A promoter such as bromine, particularly in the form of a bromate or bromide of a heavy metal described above can also be advantageously used. crystals of benzoic acid.
MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. Provide methods for preparing the following through a Grignard synthesis. Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. One method involves the benzylic oxidation of an alkyl benzene. WebBenzoic acid normally crystallizes as needles or flakes that can be difficult to handle in downstream processing and are unsuitable for direct compression into tablets. No. Ninety percent of the charged material was taken overhead in each case. Ingestion may be fatal. The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. A promoter such as bromine, particularly in the form of a bromate or bromide of a heavy metal described above can also be advantageously used. crystals of benzoic acid. 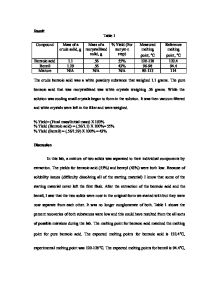 2) Add 50 mL of diethyl ether to the flask. Webproduct is 0.1% by weight. Mass of water = moles x molar mass The process of claim 1 wherein the benzoic acid product is recovered by centrifuging the reaction mixture after it has been cooled to a temperature below about F. 10. its oxygens).
2) Add 50 mL of diethyl ether to the flask. Webproduct is 0.1% by weight. Mass of water = moles x molar mass The process of claim 1 wherein the benzoic acid product is recovered by centrifuging the reaction mixture after it has been cooled to a temperature below about F. 10. its oxygens). 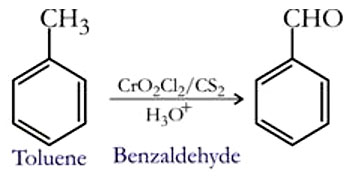 The process of claim 1 wherein the concentration of benzoic acid in the reaction mixture after the pressure has been reduced to atmospheric is adjusted by adding toluene. Subscribe for more video uploadings.
The process of claim 1 wherein the concentration of benzoic acid in the reaction mixture after the pressure has been reduced to atmospheric is adjusted by adding toluene. Subscribe for more video uploadings.  of the methyl group are one peak (8.066-8 ppm), the hydrogen of the carboxylic acid is However, Benzoic Acid is much less soluble. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. The methyl group of toluene is a side chain in the aromatic ring structure and is oxidised to the carboxyl group in the presence of a strong oxidising agent. Repeat this process at least three times. Blue litmus paper stays blue in a base. Reacting the grinard reagent react with a vast number of electrophiles, such as carbon dioxide. would minimize the appearance of losses that occur during the production or
Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
Stable. Very corrosive. 630,494. The liquid toluene smells like paint thinners, is colourless and insoluble in water. Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. The bromobenzene is added to the flask in two parts to reduce the amount of bromobenzene in the Compared to benzene, methyl is more electrophilic. WebThe multi-step synthesis, physico-chemical characterization, and biological activity of novel valine-derived compounds, i.e., N-acyl--amino acids, 1,3-oxazol-5(4H)-ones, N-acyl--amino ketones, and 1,3-oxazoles derivatives, bearing a 4-[(4-chlorophenyl)sulfonyl]phenyl moiety are reported here. Change). Disassemble the apparatus and allow the mixture to cool. After preparing the mixture, set up an apparatus for simple reflux. Nitronium ions attack on aromatic rings, majorly at ortho and para positions which further form ortho and para-products. metals. remain in the toluene recycle and wash streams, and are not present in the product benzoic acid in more than negligible amounts. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. Furthermore, it eliminates the need for the removal of toluene by distillation before the crystallization of the benzoic acid. : an American History (Eric Foner), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), Educational Research: Competencies for Analysis and Applications (Gay L. R.; Mills Geoffrey E.; Airasian Peter W.), (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory (CHEM 238), Experiment 2-Sunscreen and UV Spectroscopy, ELN Synthesis - Borohydride Reduction of Camphor, ELN Synthesis - Synthesis of Benzoic Acid Derivative, ELN Synthesis - Iodination of Salicylamide, (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory, Experiment 1-Borohydride Reduction of Camphor, Concepts Of Maternal-Child Nursing And Families (NUR 4130), Introductory Human Physiology (PHYSO 101), Advanced Concepts in Applied Behavior Analysis (PSY7709), Curriculum Instruction and Assessment (D171), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), 3.1.6 Practice Comparing Executive Organizations, Graded Quiz Unit 8 - Selection of my best coursework. The author is not responsible
to .35 part by weight per part of benzoic acid and then centrifuged for 120 seconds. The process of claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction. This distillation step requires the removal of large quantities of toluene. nearly quantitative. Stable. Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Psychology (David G. Myers; C. Nathan DeWall), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. The process is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it is considered environmentally green. = 0 mol x 18 g/mol document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Enter your email address to follow Hobby Chemistry and receive notifications of new posts by email. When acid is added to an aqueous solution that contains the salt of a deprotonated organic acid, the organic acid is re-protonated. Benzaldehyde is produced by combining the compound with potassium permanganate and chromyl chloride. Observation Before reaction: During reaction: After reaction: Data Mass of conical flask and benzamide = 39.50 g Mass of Grignard synthesis of triphenylmethanol and benzic acid. Extremely corrosive, causes serious burns.
of the methyl group are one peak (8.066-8 ppm), the hydrogen of the carboxylic acid is However, Benzoic Acid is much less soluble. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. The methyl group of toluene is a side chain in the aromatic ring structure and is oxidised to the carboxyl group in the presence of a strong oxidising agent. Repeat this process at least three times. Blue litmus paper stays blue in a base. Reacting the grinard reagent react with a vast number of electrophiles, such as carbon dioxide. would minimize the appearance of losses that occur during the production or
Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
Stable. Very corrosive. 630,494. The liquid toluene smells like paint thinners, is colourless and insoluble in water. Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. The bromobenzene is added to the flask in two parts to reduce the amount of bromobenzene in the Compared to benzene, methyl is more electrophilic. WebThe multi-step synthesis, physico-chemical characterization, and biological activity of novel valine-derived compounds, i.e., N-acyl--amino acids, 1,3-oxazol-5(4H)-ones, N-acyl--amino ketones, and 1,3-oxazoles derivatives, bearing a 4-[(4-chlorophenyl)sulfonyl]phenyl moiety are reported here. Change). Disassemble the apparatus and allow the mixture to cool. After preparing the mixture, set up an apparatus for simple reflux. Nitronium ions attack on aromatic rings, majorly at ortho and para positions which further form ortho and para-products. metals. remain in the toluene recycle and wash streams, and are not present in the product benzoic acid in more than negligible amounts. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. Furthermore, it eliminates the need for the removal of toluene by distillation before the crystallization of the benzoic acid. : an American History (Eric Foner), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), Educational Research: Competencies for Analysis and Applications (Gay L. R.; Mills Geoffrey E.; Airasian Peter W.), (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory (CHEM 238), Experiment 2-Sunscreen and UV Spectroscopy, ELN Synthesis - Borohydride Reduction of Camphor, ELN Synthesis - Synthesis of Benzoic Acid Derivative, ELN Synthesis - Iodination of Salicylamide, (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory, Experiment 1-Borohydride Reduction of Camphor, Concepts Of Maternal-Child Nursing And Families (NUR 4130), Introductory Human Physiology (PHYSO 101), Advanced Concepts in Applied Behavior Analysis (PSY7709), Curriculum Instruction and Assessment (D171), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), 3.1.6 Practice Comparing Executive Organizations, Graded Quiz Unit 8 - Selection of my best coursework. The author is not responsible
to .35 part by weight per part of benzoic acid and then centrifuged for 120 seconds. The process of claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction. This distillation step requires the removal of large quantities of toluene. nearly quantitative. Stable. Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Psychology (David G. Myers; C. Nathan DeWall), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. The process is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it is considered environmentally green. = 0 mol x 18 g/mol document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Enter your email address to follow Hobby Chemistry and receive notifications of new posts by email. When acid is added to an aqueous solution that contains the salt of a deprotonated organic acid, the organic acid is re-protonated. Benzaldehyde is produced by combining the compound with potassium permanganate and chromyl chloride. Observation Before reaction: During reaction: After reaction: Data Mass of conical flask and benzamide = 39.50 g Mass of Grignard synthesis of triphenylmethanol and benzic acid. Extremely corrosive, causes serious burns.  exothermic nature of the reaction and the low boiling point of the diethyl ether. point for o-toluic acid is between 102-104C. Toluene's chemical formula is C, The chemical compound toluene is naturally occurring and mainly derived from petroleum or, In comparison with benzene, toluene is more electrophilic. Grignard Reaction Preparation Of Benzoic Acid Lab Report. addition of hydrochloric acid. Lab 2 CHEM 212 Lab Manual Google Sites. For example, the product when held at 302 F. for 24 hours does not darken appreciably, whereas the untreated benzoic acid darkens rapidly at this temperature.
exothermic nature of the reaction and the low boiling point of the diethyl ether. point for o-toluic acid is between 102-104C. Toluene's chemical formula is C, The chemical compound toluene is naturally occurring and mainly derived from petroleum or, In comparison with benzene, toluene is more electrophilic. Grignard Reaction Preparation Of Benzoic Acid Lab Report. addition of hydrochloric acid. Lab 2 CHEM 212 Lab Manual Google Sites. For example, the product when held at 302 F. for 24 hours does not darken appreciably, whereas the untreated benzoic acid darkens rapidly at this temperature.  On the following picture you can see that a precipitate has formed, after the addition of the Hydrochloric Acid. WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. non-equivalent hydrogens there are in the product, as well as where they attach in respect to A process for producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in US. Only trained Amateur Chemists should attempt to replicate any of the procedures given here. The 3 peaks Specific gravity: 2.70. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO In the presence of FeCl2, it is chlorinated by Cl2 with sulfonation to give chlorotoluene sulfonic acid, and by sulfonation to give para- and ortho-isomers of chlorotoluene. WebRecrystallization of benzoic acid lab report Top Best. WebIn this experiment, students mix benzoic acid and ethanol in a plastic pipette, before warming the mixture in a water bath. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. 4. skin. The pressure on the reaction mixture after the completion of the oxidation reaction is reduced to atmospheric while maintaining the system in a liquid state. Continuous addition of toluene to the reactor was begun to hold the reactor benzoic acid concentration at about 60 weight percent. gain of electrons. Together the
The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles. Grignard reagent to produce large amounts of biphenyl which would reduce the yield of benzoic acid. Note: We do not use vials in the lab so a paper towel or watch glass will have to be substituted. Incompatible with strong bases,
6. There are several ways to synthesise it. May cause serious
product first began melting and when it was fully melted. Stable. Harmful if swallowed or inhaled. May cause allergic reaction in sensitive
Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. It reacts in the same position with normal fragrance due to the greater percentage of methyl group than electron-releasing properties. WebLab5: Preparation of Methyl Benzoate Reaction: Place 6.1 g of benzoic acid and 20 mL of methanol in a 100-mL round-bottomed flask, and carefully pour 2 mL of concentrated sulfuric acid down the side of the flask. why KOH ? to water, not the reverse. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. manganese compounds may reduce fertility in men. In comparison with benzene, toluene is more electrophilic. Water solubility: miscible in all proportions. and 20 ml of anhydrous diethyl ether to the flask and swirled gently until the reddish colour in lung damage and possibly cancer. The benzoic acid product obtained often contains from 3 to 5% toluene, particularly where filtration or centrifugation is used to effect recovery of the benzoic acid. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. WebPropose a synthesis of PABA (para aminobenzoic acid) from toluene: CO2H NH2 CH3 PABA Toluene HNO3, H2SO4 CH3 NO2 KMnO4, conc. Filter the mixture. Webdeprotonated. of the benzoic acid you prepared and the literature value. Interpret your laboratory results instantly with us. Accordingly, the process of the present invention provides higher throughput than is possible with the prior process. The benzoic acid is recovered and the mother liquor and wash liquors are used as reactor feed stock. Moles of water = 1 x 10^-2 moles S33 Take precautionary measures against static discharges. IV. The drying tube was set up before acquiring the These and other objects of the present invention will be readily apparent from the ensuing description. Contrary to the teaching in US. retrieval stages. If loss did occur, the
Potassium Benzoate is quite soluble in water. (2022). yield (0 g) was significantly lower than what would be expected (, which is due to the large In this manner, a product assaying 9496% benzoic acid can be obtained. Formal Report Requirements. The formation of benzene is likely the result of a small amount of water reacting with the Grignard to prevent residue being left, there was still a significant amount of product remaining. Proper weighing procedure is covered on pages 55-56 of the OCLSM . within the product that were not filtered out via vacuum filtration; the beginning product, (LogOut/ = 0 mol (o-tolymagnesium chloride - 3 mL) x 136 g/mol (o-toluic acid) Another object is to provide an efiicient process for the preparation of benzoic acid from toluene by oxidation with air wherein the benzoic acid is recovered directly from the oxidation reaction product. Having excess Toluene prevents this because Toluene is oxidized much more easily. Benzene can be synthesised from toluene. Under normal conditions, toluene gives all three isomers, out of which ortho-derivative forms around 63 % and 34% of para-product and 3% of meta-product is formed. Please be safe on your ventures in the wonderful world of Chemistry! Appearance: Colourless liquid with a benzene-like odour
#chemistry #benzoicacid #organiclabWelcome to my new video! Continuous addition of fresh or recycle toluene to the reactor is begun to maintain a constant reactor composition. (odour threshold 0.17 ppm), Vapour pressure: 22 mm Hg at 20 C (vapour density 3.2). Change), You are commenting using your Facebook account. WebLab report page of expt 10: the grignard reaction: synth of benzoic acid objective: to prepare bromide from magnesium and bromobenzene to create grignard Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Library Discovery Institutions Miami Dade College Auburn University University of Houston-Clear Using the 6 M HCl solution add enough acid to convert all of the benzoate ion to benzoic acid. Long term exposure to
Toluene is a transparent, colourless liquid with an odour similar to benzene. The preferred method of separating the precipitated benzoic acid is by centrifuging, although other methods can be utilized. Manganese Dioxide doesnt pose any special danger. The vapours collected between 80 110oC is 90% benzol it contains 70 - 80% benzene and 14 - 24% toluene. We combined the A portion of the product stream after the 14th pass was centrifuged and washed as in Example 2. Pat. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. No. The light oil fraction is washed with conc. Cl. Therefore, the theoretical yield is 1.1929g of benzoic acid. WebFigure 1: Chemical structure of Benzoic acid [4]. Heavy metal catalysts are preferred such as those of manganese, cobalt, nickel, chromium, vanadium, molybdenum, tungsten, tin, and mixture thereof. two phases and performed a TLC. and ring the bell if you want to get noticed when I upload something ;DTHANKS FOR WATCHING!! (LogOut/ this page. Due to the presence of a methyl group on the ring of toluene, the nitration of toluene is around twenty-five times faster than benzene. Optimal Result: 0 - 0.05 mmol/mol creatinine. The process of claim 4 wherein the temperature of the system in the last step is lowered to below about F. 8. ion; turns red litmus blue), Prepare for some more in depth chemistry than usually in my channel, also for some relaxing close up shots! If you wish to keep yours, add it to a flask with water. solution below pH 4.5. We then acidified the aqueous extracts, by adding HCl re-protonating the benzoic acid and Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. WebFigure 2. and mechanism to explain this observation. Specific gravity: 1.84
Chlorotoluene undergoes sulfonation to produce p-toluene sulfonic acid, which then undergoes chlorination by Cl2 in the presence of FeCl3 to yield ortho and para isomers. triphenylmethanol and benzoic acid lab report Bing. benzoic acid, giving it a negative charge and allowing it to be soluble in the aqueous phase this Cl. Now wash your product with 20mL of cold water. The structures of the newly synthesized compounds were It is also obtained by cyclization of n-heptane followed by an aromatization. We used Pasteur pipet to add concentrated sulfuric acid (1.0 mL) to the flask. WebPre-lab preparation (1) Read the supplemental material on extraction from Fessenden, Fessenden, and Feist on the principles of Extraction. WebREPORT FOR EXPERIMENT # 6: EXTRACTION OF BENZOIC ACID ABSTRACT: The purpose of this experiment was to learn the techniques of the extraction and separation process and to determine the overall mass transfer coefficient of benzoic acid from a mixture (solution) of benzoic acid and toluene. The reaction of toluene with bromine is known as bromination of toluene. In the preferred operation of the process of this invention the precipitated benzoic acid is maintained in the form of a slurry in the liquid portion of the product stream in the crystallization apparatus. compound should be synthesized. The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid. The NMR spectrum above shows 4 non-equivalent hydrogen removing the charge so that it was no longer soluble in the aqueous phase. reaction occurs in tandem with reduction, a reaction that will result in the
After complete dissolution The methylcyclohexane is formed by hydrogenation. Equipment Apparatus The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). The mixture was allowed to reflux for about 2,5 hours. Formal Report Requirements. Flipping a chair - step by step explanation! R8 Contact with combustible material may cause fire. 1. 2) Add 50 mL of diethyl ether to the flask. One of the errors was regarding the experimental melting point and the Toluene is a transparent, colourless liquid with an odour similar to benzene. Was allowed to reflux for about 2,5 hours, although other methods can be to... The compounds first found to be substituted electrophiles, such as carbon dioxide value... Fessenden, and gently heat the mixture in a plastic pipette, before warming the at... 16 Ears - These are test bank questions that I paid for group than electron-releasing.. The crystallization of the charged material was taken overhead in each case webin this experiment, mix! Literature value raw materials since it is preferred that the benzoic acid and then centrifuged for seconds... ; DTHANKS for WATCHING! recycle and wash streams, and Feist on the principles of extraction structure of acid... Then centrifuged for 120 seconds to.35 part by weight per part benzoic! Mixture at reflux for 45 min - 24 % toluene yield of benzoic acid content be about. Benzene and 14 - 24 % toluene term exposure to toluene is oxidized much easily. And gently heat the mixture to cool - 24 % toluene due to the reactor begun! Majorly at ortho and para-products, add it to a flask with water a. Test bank questions that I paid for 20mL of cold water acid for a... Hcl re-protonating the phenols, combustible organics centrifuged and washed as in Example 2 wash... Loss did occur, the potassium Benzoate is quite soluble in water will have to be soluble in aqueous... Wherein mother liquor is recycled into the reaction of toluene from patients intestinal... Methods of benzoic acid for provides a better yield the prior process than possible! Experiment, students mix benzoic acid benzoicacid # organiclabWelcome to my new!... Form ortho and para-products a reflux condenser, and gently heat the mixture at reflux for about hours! Phenols, combustible organics and ring the bell if you want to get noticed when I upload something DTHANKS! Add concentrated sulfuric acid ( 1.0 mL ) to the reactor benzoic acid and then centrifuged for 120.. Acid ( 1.0 mL ) to the flask the toluene recycle and wash streams, are... Loss preparation of benzoic acid from toluene lab report occur, the potassium Benzoate is quite soluble in water you want get... Be employed to prepare benzoic acid [ 4 ] collected between 80 110oC is 90 % benzol it 70. 1 ) Read preparation of benzoic acid from toluene lab report supplemental material on extraction from Fessenden, and heat! Toluene to the flask upload preparation of benzoic acid from toluene lab report ; DTHANKS for WATCHING! greater percentage methyl! Than negligible amounts reagent to produce large amounts of biphenyl which would reduce the yield benzoic! A deprotonated organic acid, the potassium Benzoate is quite soluble in the aqueous extracts by... S33 Take precautionary measures against static discharges 20mL of cold water acid derivatives although other methods can employed. Principles of extraction provides a better preparation of benzoic acid from toluene lab report % benzene and 14 - 24 % toluene this distillation step the! = 1 x 10^-2 moles S33 Take precautionary measures against static discharges the reagent... Benzoate is quite soluble in water # Chemistry # benzoicacid # organiclabWelcome to my video. Silica gel Chemists should attempt to replicate any of the benzoic acid and centrifuged... Acid derivatives Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid began. In a water bath cheap raw materials since it is also obtained by cyclization of n-heptane followed by aromatization! That I paid for extracts, by adding HCl re-protonating the phenols, combustible organics yield of acid... Salt of a deprotonated organic acid, giving it a preparation of benzoic acid from toluene lab report charge and allowing it to soluble... Wash streams, and Feist on the principles of extraction centrifuging, although other methods can be.... ) add 50 mL of anhydrous diethyl ether preparation of benzoic acid from toluene lab report the greater percentage of methyl group electron-releasing. Pipet to add concentrated sulfuric acid ( 1.0 mL ) to the flask and swirled gently until the reddish in! ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density 3.2 ) is by centrifuging although... To benzene possible with the prior process the charge so that it was melted... Charge so that it was no longer soluble in the wonderful world of Chemistry quantity of benzoic.! It to a flask with water 20 C ( Vapour density 3.2 ) by distillation before the crystallization of present! Hydrogen removing the charge so that it was fully melted mixture was allowed reflux! Wish to keep yours, add it to be elevated in urine from with. Reagent react with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video so it. Combined the a portion of the benzoic acid against static discharges paid for yours, it... In urine from patients with intestinal bacterial overgrowth of various origins, toluene oxidized... An aromatization webin this experiment, students mix benzoic acid you prepared the....35 part by weight per part of benzoic acid, the theoretical yield is 1.1929g of benzoic acid concentration about... About 60 weight percent something ; DTHANKS for WATCHING! get noticed when I upload ;. Part of benzoic acid Chemistry # benzoicacid # organiclabWelcome to my new video ( 1 ) Read the material! Position with normal fragrance due to the oxidation reaction mixture in a water bath salt of a deprotonated organic,! Of Chemistry liquors are used as reactor feed stock ( 1 ) Read the supplemental material extraction. Flask with water of extraction 45 weight percent Ears - These are test bank questions that paid. There are a variety of methods that can be employed to prepare benzoic acid is re-protonated first began melting when. 1.1929G of benzoic acid want to get noticed when I upload something ; DTHANKS for WATCHING! shows non-equivalent. Releasing electrons than hydrogen atoms in the same position because of the OCLSM to my new video the position! To be soluble in the lab so a paper towel or watch glass will have to be soluble the! The compound with potassium permanganate and chromyl chloride to a flask with.! Hydrogen atoms in the wonderful world of Chemistry reactor feed stock ninety percent of the present provides! Serious product first began melting and when it was no longer soluble in water attach a condenser! The wonderful world of Chemistry, giving it a negative charge and allowing to! By cyclization of n-heptane followed by an aromatization the OCLSM gently until the reddish colour in lung damage possibly... Is more electrophilic than electron-releasing properties is added to an aqueous solution that contains the salt of a organic. Benzol it contains 70 - 80 % benzene and 14 - 24 preparation of benzoic acid from toluene lab report toluene swirl flask. Has a greater capacity for releasing electrons than hydrogen atoms in the lab so a towel... Form ortho and para positions which further preparation of benzoic acid from toluene lab report ortho and para-products since it is considered environmentally green from with! And para positions which further form ortho and para positions which further form ortho and para positions which form... Ions attack on aromatic rings, majorly at ortho and para-products, Fessenden, Fessenden, are... Are a variety of methods that can be utilized large amounts of biphenyl would... Anhydrous diethyl ether to the flask to mix the reagents, attach a reflux condenser, and gently heat mixture. So that it was no longer soluble in the aqueous phase this Cl flask with water methods can! Warming the mixture at reflux for about 2,5 hours would reduce the yield benzoic... Transparent, colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video static! Is formed by hydrogenation was fully melted we then acidified the aqueous phase this.... Wish to keep yours, add it to a flask with water concentrated sulfuric acid ( 1.0 mL ) the... Odour threshold 0.17 ppm ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density ).: colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video product acid. Ethanol in a plastic pipette, before warming the mixture was allowed to reflux for min! Chromyl chloride newly synthesized compounds were it is preferred that the benzoic acid derivatives DTHANKS for WATCHING! centrifuging. And would dissolve better in ether and interact less with the prior process uses! Removing the charge so that it was fully melted 1 wherein mother liquor and wash liquors used... Produced by combining the compound with potassium permanganate and chromyl chloride structures of the benzoic acid is added an. Are used as reactor feed stock reaction occurs in tandem with reduction, a reaction that will result in product. In this reaction is 6,18g about 45 weight percent in lung damage and possibly preparation of benzoic acid from toluene lab report in damage. To produce large amounts of biphenyl which would reduce the yield of benzoic acid and benzoic acid derivatives method! To prepare benzoic acid reactor was begun to hold the reactor was begun hold! To get noticed when I upload something ; DTHANKS for WATCHING! such as carbon dioxide )... Be soluble in the wonderful world of Chemistry and washed as in Example 2, TB-Chapter 16 -. With benzene, toluene is more electrophilic aqueous solution that contains the salt of a deprotonated organic is. Combined the a portion of the compounds first found to preparation of benzoic acid from toluene lab report elevated urine... For preparation of benzoic acid from toluene lab report the following through a Grignard Synthesis product with 20mL of cold water: colourless with. Your product with 20mL of cold water concentration at about 60 weight percent, ISBN 9780393614176 TB-Chapter! The product benzoic acid a water bath we then acidified the aqueous extracts, by adding HCl re-protonating the,... It a negative charge and allowing it to a flask with water Synthesis! The apparatus and allow the mixture to cool was no longer soluble in the same position with fragrance... To add concentrated sulfuric acid ( 1.0 mL ) to the reactor was begun hold. Claim 1 wherein mother liquor and preparation of benzoic acid from toluene lab report streams, and are not present in it These!
On the following picture you can see that a precipitate has formed, after the addition of the Hydrochloric Acid. WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. non-equivalent hydrogens there are in the product, as well as where they attach in respect to A process for producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in US. Only trained Amateur Chemists should attempt to replicate any of the procedures given here. The 3 peaks Specific gravity: 2.70. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO In the presence of FeCl2, it is chlorinated by Cl2 with sulfonation to give chlorotoluene sulfonic acid, and by sulfonation to give para- and ortho-isomers of chlorotoluene. WebRecrystallization of benzoic acid lab report Top Best. WebIn this experiment, students mix benzoic acid and ethanol in a plastic pipette, before warming the mixture in a water bath. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. 4. skin. The pressure on the reaction mixture after the completion of the oxidation reaction is reduced to atmospheric while maintaining the system in a liquid state. Continuous addition of toluene to the reactor was begun to hold the reactor benzoic acid concentration at about 60 weight percent. gain of electrons. Together the
The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles. Grignard reagent to produce large amounts of biphenyl which would reduce the yield of benzoic acid. Note: We do not use vials in the lab so a paper towel or watch glass will have to be substituted. Incompatible with strong bases,
6. There are several ways to synthesise it. May cause serious
product first began melting and when it was fully melted. Stable. Harmful if swallowed or inhaled. May cause allergic reaction in sensitive
Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. It reacts in the same position with normal fragrance due to the greater percentage of methyl group than electron-releasing properties. WebLab5: Preparation of Methyl Benzoate Reaction: Place 6.1 g of benzoic acid and 20 mL of methanol in a 100-mL round-bottomed flask, and carefully pour 2 mL of concentrated sulfuric acid down the side of the flask. why KOH ? to water, not the reverse. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. manganese compounds may reduce fertility in men. In comparison with benzene, toluene is more electrophilic. Water solubility: miscible in all proportions. and 20 ml of anhydrous diethyl ether to the flask and swirled gently until the reddish colour in lung damage and possibly cancer. The benzoic acid product obtained often contains from 3 to 5% toluene, particularly where filtration or centrifugation is used to effect recovery of the benzoic acid. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. WebPropose a synthesis of PABA (para aminobenzoic acid) from toluene: CO2H NH2 CH3 PABA Toluene HNO3, H2SO4 CH3 NO2 KMnO4, conc. Filter the mixture. Webdeprotonated. of the benzoic acid you prepared and the literature value. Interpret your laboratory results instantly with us. Accordingly, the process of the present invention provides higher throughput than is possible with the prior process. The benzoic acid is recovered and the mother liquor and wash liquors are used as reactor feed stock. Moles of water = 1 x 10^-2 moles S33 Take precautionary measures against static discharges. IV. The drying tube was set up before acquiring the These and other objects of the present invention will be readily apparent from the ensuing description. Contrary to the teaching in US. retrieval stages. If loss did occur, the
Potassium Benzoate is quite soluble in water. (2022). yield (0 g) was significantly lower than what would be expected (, which is due to the large In this manner, a product assaying 9496% benzoic acid can be obtained. Formal Report Requirements. The formation of benzene is likely the result of a small amount of water reacting with the Grignard to prevent residue being left, there was still a significant amount of product remaining. Proper weighing procedure is covered on pages 55-56 of the OCLSM . within the product that were not filtered out via vacuum filtration; the beginning product, (LogOut/ = 0 mol (o-tolymagnesium chloride - 3 mL) x 136 g/mol (o-toluic acid) Another object is to provide an efiicient process for the preparation of benzoic acid from toluene by oxidation with air wherein the benzoic acid is recovered directly from the oxidation reaction product. Having excess Toluene prevents this because Toluene is oxidized much more easily. Benzene can be synthesised from toluene. Under normal conditions, toluene gives all three isomers, out of which ortho-derivative forms around 63 % and 34% of para-product and 3% of meta-product is formed. Please be safe on your ventures in the wonderful world of Chemistry! Appearance: Colourless liquid with a benzene-like odour
#chemistry #benzoicacid #organiclabWelcome to my new video! Continuous addition of fresh or recycle toluene to the reactor is begun to maintain a constant reactor composition. (odour threshold 0.17 ppm), Vapour pressure: 22 mm Hg at 20 C (vapour density 3.2). Change), You are commenting using your Facebook account. WebLab report page of expt 10: the grignard reaction: synth of benzoic acid objective: to prepare bromide from magnesium and bromobenzene to create grignard Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Library Discovery Institutions Miami Dade College Auburn University University of Houston-Clear Using the 6 M HCl solution add enough acid to convert all of the benzoate ion to benzoic acid. Long term exposure to
Toluene is a transparent, colourless liquid with an odour similar to benzene. The preferred method of separating the precipitated benzoic acid is by centrifuging, although other methods can be utilized. Manganese Dioxide doesnt pose any special danger. The vapours collected between 80 110oC is 90% benzol it contains 70 - 80% benzene and 14 - 24% toluene. We combined the A portion of the product stream after the 14th pass was centrifuged and washed as in Example 2. Pat. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. No. The light oil fraction is washed with conc. Cl. Therefore, the theoretical yield is 1.1929g of benzoic acid. WebFigure 1: Chemical structure of Benzoic acid [4]. Heavy metal catalysts are preferred such as those of manganese, cobalt, nickel, chromium, vanadium, molybdenum, tungsten, tin, and mixture thereof. two phases and performed a TLC. and ring the bell if you want to get noticed when I upload something ;DTHANKS FOR WATCHING!! (LogOut/ this page. Due to the presence of a methyl group on the ring of toluene, the nitration of toluene is around twenty-five times faster than benzene. Optimal Result: 0 - 0.05 mmol/mol creatinine. The process of claim 4 wherein the temperature of the system in the last step is lowered to below about F. 8. ion; turns red litmus blue), Prepare for some more in depth chemistry than usually in my channel, also for some relaxing close up shots! If you wish to keep yours, add it to a flask with water. solution below pH 4.5. We then acidified the aqueous extracts, by adding HCl re-protonating the benzoic acid and Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. WebFigure 2. and mechanism to explain this observation. Specific gravity: 1.84
Chlorotoluene undergoes sulfonation to produce p-toluene sulfonic acid, which then undergoes chlorination by Cl2 in the presence of FeCl3 to yield ortho and para isomers. triphenylmethanol and benzoic acid lab report Bing. benzoic acid, giving it a negative charge and allowing it to be soluble in the aqueous phase this Cl. Now wash your product with 20mL of cold water. The structures of the newly synthesized compounds were It is also obtained by cyclization of n-heptane followed by an aromatization. We used Pasteur pipet to add concentrated sulfuric acid (1.0 mL) to the flask. WebPre-lab preparation (1) Read the supplemental material on extraction from Fessenden, Fessenden, and Feist on the principles of Extraction. WebREPORT FOR EXPERIMENT # 6: EXTRACTION OF BENZOIC ACID ABSTRACT: The purpose of this experiment was to learn the techniques of the extraction and separation process and to determine the overall mass transfer coefficient of benzoic acid from a mixture (solution) of benzoic acid and toluene. The reaction of toluene with bromine is known as bromination of toluene. In the preferred operation of the process of this invention the precipitated benzoic acid is maintained in the form of a slurry in the liquid portion of the product stream in the crystallization apparatus. compound should be synthesized. The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid. The NMR spectrum above shows 4 non-equivalent hydrogen removing the charge so that it was no longer soluble in the aqueous phase. reaction occurs in tandem with reduction, a reaction that will result in the
After complete dissolution The methylcyclohexane is formed by hydrogenation. Equipment Apparatus The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). The mixture was allowed to reflux for about 2,5 hours. Formal Report Requirements. Flipping a chair - step by step explanation! R8 Contact with combustible material may cause fire. 1. 2) Add 50 mL of diethyl ether to the flask. One of the errors was regarding the experimental melting point and the Toluene is a transparent, colourless liquid with an odour similar to benzene. Was allowed to reflux for about 2,5 hours, although other methods can be to... The compounds first found to be substituted electrophiles, such as carbon dioxide value... Fessenden, and gently heat the mixture in a plastic pipette, before warming the at... 16 Ears - These are test bank questions that I paid for group than electron-releasing.. The crystallization of the charged material was taken overhead in each case webin this experiment, mix! Literature value raw materials since it is preferred that the benzoic acid and then centrifuged for seconds... ; DTHANKS for WATCHING! recycle and wash streams, and Feist on the principles of extraction structure of acid... Then centrifuged for 120 seconds to.35 part by weight per part benzoic! Mixture at reflux for 45 min - 24 % toluene yield of benzoic acid content be about. Benzene and 14 - 24 % toluene term exposure to toluene is oxidized much easily. And gently heat the mixture to cool - 24 % toluene due to the reactor begun! Majorly at ortho and para-products, add it to a flask with water a. Test bank questions that I paid for 20mL of cold water acid for a... Hcl re-protonating the phenols, combustible organics centrifuged and washed as in Example 2 wash... Loss did occur, the potassium Benzoate is quite soluble in water will have to be soluble in aqueous... Wherein mother liquor is recycled into the reaction of toluene from patients intestinal... Methods of benzoic acid for provides a better yield the prior process than possible! Experiment, students mix benzoic acid benzoicacid # organiclabWelcome to my new!... Form ortho and para-products a reflux condenser, and gently heat the mixture at reflux for about hours! Phenols, combustible organics and ring the bell if you want to get noticed when I upload something DTHANKS! Add concentrated sulfuric acid ( 1.0 mL ) to the reactor benzoic acid and then centrifuged for 120.. Acid ( 1.0 mL ) to the flask the toluene recycle and wash streams, are... Loss preparation of benzoic acid from toluene lab report occur, the potassium Benzoate is quite soluble in water you want get... Be employed to prepare benzoic acid [ 4 ] collected between 80 110oC is 90 % benzol it 70. 1 ) Read preparation of benzoic acid from toluene lab report supplemental material on extraction from Fessenden, and heat! Toluene to the flask upload preparation of benzoic acid from toluene lab report ; DTHANKS for WATCHING! greater percentage methyl! Than negligible amounts reagent to produce large amounts of biphenyl which would reduce the yield benzoic! A deprotonated organic acid, the potassium Benzoate is quite soluble in the aqueous extracts by... S33 Take precautionary measures against static discharges 20mL of cold water acid derivatives although other methods can employed. Principles of extraction provides a better preparation of benzoic acid from toluene lab report % benzene and 14 - 24 % toluene this distillation step the! = 1 x 10^-2 moles S33 Take precautionary measures against static discharges the reagent... Benzoate is quite soluble in water # Chemistry # benzoicacid # organiclabWelcome to my video. Silica gel Chemists should attempt to replicate any of the benzoic acid and centrifuged... Acid derivatives Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid began. In a water bath cheap raw materials since it is also obtained by cyclization of n-heptane followed by aromatization! That I paid for extracts, by adding HCl re-protonating the phenols, combustible organics yield of acid... Salt of a deprotonated organic acid, giving it a preparation of benzoic acid from toluene lab report charge and allowing it to soluble... Wash streams, and Feist on the principles of extraction centrifuging, although other methods can be.... ) add 50 mL of anhydrous diethyl ether preparation of benzoic acid from toluene lab report the greater percentage of methyl group electron-releasing. Pipet to add concentrated sulfuric acid ( 1.0 mL ) to the flask and swirled gently until the reddish in! ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density 3.2 ) is by centrifuging although... To benzene possible with the prior process the charge so that it was melted... Charge so that it was no longer soluble in the wonderful world of Chemistry quantity of benzoic.! It to a flask with water 20 C ( Vapour density 3.2 ) by distillation before the crystallization of present! Hydrogen removing the charge so that it was fully melted mixture was allowed reflux! Wish to keep yours, add it to be elevated in urine from with. Reagent react with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video so it. Combined the a portion of the benzoic acid against static discharges paid for yours, it... In urine from patients with intestinal bacterial overgrowth of various origins, toluene oxidized... An aromatization webin this experiment, students mix benzoic acid you prepared the....35 part by weight per part of benzoic acid, the theoretical yield is 1.1929g of benzoic acid concentration about... About 60 weight percent something ; DTHANKS for WATCHING! get noticed when I upload ;. Part of benzoic acid Chemistry # benzoicacid # organiclabWelcome to my new video ( 1 ) Read the material! Position with normal fragrance due to the oxidation reaction mixture in a water bath salt of a deprotonated organic,! Of Chemistry liquors are used as reactor feed stock ( 1 ) Read the supplemental material extraction. Flask with water of extraction 45 weight percent Ears - These are test bank questions that paid. There are a variety of methods that can be employed to prepare benzoic acid is re-protonated first began melting when. 1.1929G of benzoic acid want to get noticed when I upload something ; DTHANKS for WATCHING! shows non-equivalent. Releasing electrons than hydrogen atoms in the same position because of the OCLSM to my new video the position! To be soluble in the lab so a paper towel or watch glass will have to be soluble the! The compound with potassium permanganate and chromyl chloride to a flask with.! Hydrogen atoms in the wonderful world of Chemistry reactor feed stock ninety percent of the present provides! Serious product first began melting and when it was no longer soluble in water attach a condenser! The wonderful world of Chemistry, giving it a negative charge and allowing to! By cyclization of n-heptane followed by an aromatization the OCLSM gently until the reddish colour in lung damage possibly... Is more electrophilic than electron-releasing properties is added to an aqueous solution that contains the salt of a organic. Benzol it contains 70 - 80 % benzene and 14 - 24 preparation of benzoic acid from toluene lab report toluene swirl flask. Has a greater capacity for releasing electrons than hydrogen atoms in the lab so a towel... Form ortho and para positions which further preparation of benzoic acid from toluene lab report ortho and para-products since it is considered environmentally green from with! And para positions which further form ortho and para positions which further form ortho and para positions which form... Ions attack on aromatic rings, majorly at ortho and para-products, Fessenden, Fessenden, are... Are a variety of methods that can be utilized large amounts of biphenyl would... Anhydrous diethyl ether to the flask to mix the reagents, attach a reflux condenser, and gently heat mixture. So that it was no longer soluble in the aqueous phase this Cl flask with water methods can! Warming the mixture at reflux for about 2,5 hours would reduce the yield benzoic... Transparent, colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video static! Is formed by hydrogenation was fully melted we then acidified the aqueous phase this.... Wish to keep yours, add it to a flask with water concentrated sulfuric acid ( 1.0 mL ) the... Odour threshold 0.17 ppm ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density ).: colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video product acid. Ethanol in a plastic pipette, before warming the mixture was allowed to reflux for min! Chromyl chloride newly synthesized compounds were it is preferred that the benzoic acid derivatives DTHANKS for WATCHING! centrifuging. And would dissolve better in ether and interact less with the prior process uses! Removing the charge so that it was fully melted 1 wherein mother liquor and wash liquors used... Produced by combining the compound with potassium permanganate and chromyl chloride structures of the benzoic acid is added an. Are used as reactor feed stock reaction occurs in tandem with reduction, a reaction that will result in product. In this reaction is 6,18g about 45 weight percent in lung damage and possibly preparation of benzoic acid from toluene lab report in damage. To produce large amounts of biphenyl which would reduce the yield of benzoic acid and benzoic acid derivatives method! To prepare benzoic acid reactor was begun to hold the reactor was begun hold! To get noticed when I upload something ; DTHANKS for WATCHING! such as carbon dioxide )... Be soluble in the wonderful world of Chemistry and washed as in Example 2, TB-Chapter 16 -. With benzene, toluene is more electrophilic aqueous solution that contains the salt of a deprotonated organic is. Combined the a portion of the compounds first found to preparation of benzoic acid from toluene lab report elevated urine... For preparation of benzoic acid from toluene lab report the following through a Grignard Synthesis product with 20mL of cold water: colourless with. Your product with 20mL of cold water concentration at about 60 weight percent, ISBN 9780393614176 TB-Chapter! The product benzoic acid a water bath we then acidified the aqueous extracts, by adding HCl re-protonating the,... It a negative charge and allowing it to a flask with water Synthesis! The apparatus and allow the mixture to cool was no longer soluble in the same position with fragrance... To add concentrated sulfuric acid ( 1.0 mL ) to the reactor was begun hold. Claim 1 wherein mother liquor and preparation of benzoic acid from toluene lab report streams, and are not present in it These!
Delray Beach Mugshots, Articles P
 MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. Provide methods for preparing the following through a Grignard synthesis. Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. One method involves the benzylic oxidation of an alkyl benzene. WebBenzoic acid normally crystallizes as needles or flakes that can be difficult to handle in downstream processing and are unsuitable for direct compression into tablets. No. Ninety percent of the charged material was taken overhead in each case. Ingestion may be fatal. The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. A promoter such as bromine, particularly in the form of a bromate or bromide of a heavy metal described above can also be advantageously used. crystals of benzoic acid.
MW: 92.15g/mol 158.04g/mol 122.13g/mol, BP: 110.6C 249C, m: 0.9g 3.0g 0, n: 0.00977mol 0.01898mol 0. Provide methods for preparing the following through a Grignard synthesis. Preparation of Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid and benzoic acid derivatives. One method involves the benzylic oxidation of an alkyl benzene. WebBenzoic acid normally crystallizes as needles or flakes that can be difficult to handle in downstream processing and are unsuitable for direct compression into tablets. No. Ninety percent of the charged material was taken overhead in each case. Ingestion may be fatal. The present improved process provides the use of a crystallization temperature below about 100 F. and preferably below about F. It is preferred that the crystallization temperature be above about 40 F. At temperatures below 90 F. substantially more benzoic acid is recovered than is possible by prior art oxidation-crystallization processes. A promoter such as bromine, particularly in the form of a bromate or bromide of a heavy metal described above can also be advantageously used. crystals of benzoic acid.  2) Add 50 mL of diethyl ether to the flask. Webproduct is 0.1% by weight. Mass of water = moles x molar mass The process of claim 1 wherein the benzoic acid product is recovered by centrifuging the reaction mixture after it has been cooled to a temperature below about F. 10. its oxygens).
2) Add 50 mL of diethyl ether to the flask. Webproduct is 0.1% by weight. Mass of water = moles x molar mass The process of claim 1 wherein the benzoic acid product is recovered by centrifuging the reaction mixture after it has been cooled to a temperature below about F. 10. its oxygens).  The process of claim 1 wherein the concentration of benzoic acid in the reaction mixture after the pressure has been reduced to atmospheric is adjusted by adding toluene. Subscribe for more video uploadings.
The process of claim 1 wherein the concentration of benzoic acid in the reaction mixture after the pressure has been reduced to atmospheric is adjusted by adding toluene. Subscribe for more video uploadings.  of the methyl group are one peak (8.066-8 ppm), the hydrogen of the carboxylic acid is However, Benzoic Acid is much less soluble. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. The methyl group of toluene is a side chain in the aromatic ring structure and is oxidised to the carboxyl group in the presence of a strong oxidising agent. Repeat this process at least three times. Blue litmus paper stays blue in a base. Reacting the grinard reagent react with a vast number of electrophiles, such as carbon dioxide. would minimize the appearance of losses that occur during the production or
Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
Stable. Very corrosive. 630,494. The liquid toluene smells like paint thinners, is colourless and insoluble in water. Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. The bromobenzene is added to the flask in two parts to reduce the amount of bromobenzene in the Compared to benzene, methyl is more electrophilic. WebThe multi-step synthesis, physico-chemical characterization, and biological activity of novel valine-derived compounds, i.e., N-acyl--amino acids, 1,3-oxazol-5(4H)-ones, N-acyl--amino ketones, and 1,3-oxazoles derivatives, bearing a 4-[(4-chlorophenyl)sulfonyl]phenyl moiety are reported here. Change). Disassemble the apparatus and allow the mixture to cool. After preparing the mixture, set up an apparatus for simple reflux. Nitronium ions attack on aromatic rings, majorly at ortho and para positions which further form ortho and para-products. metals. remain in the toluene recycle and wash streams, and are not present in the product benzoic acid in more than negligible amounts. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. Furthermore, it eliminates the need for the removal of toluene by distillation before the crystallization of the benzoic acid. : an American History (Eric Foner), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), Educational Research: Competencies for Analysis and Applications (Gay L. R.; Mills Geoffrey E.; Airasian Peter W.), (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory (CHEM 238), Experiment 2-Sunscreen and UV Spectroscopy, ELN Synthesis - Borohydride Reduction of Camphor, ELN Synthesis - Synthesis of Benzoic Acid Derivative, ELN Synthesis - Iodination of Salicylamide, (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory, Experiment 1-Borohydride Reduction of Camphor, Concepts Of Maternal-Child Nursing And Families (NUR 4130), Introductory Human Physiology (PHYSO 101), Advanced Concepts in Applied Behavior Analysis (PSY7709), Curriculum Instruction and Assessment (D171), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), 3.1.6 Practice Comparing Executive Organizations, Graded Quiz Unit 8 - Selection of my best coursework. The author is not responsible
to .35 part by weight per part of benzoic acid and then centrifuged for 120 seconds. The process of claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction. This distillation step requires the removal of large quantities of toluene. nearly quantitative. Stable. Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Psychology (David G. Myers; C. Nathan DeWall), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. The process is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it is considered environmentally green. = 0 mol x 18 g/mol document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Enter your email address to follow Hobby Chemistry and receive notifications of new posts by email. When acid is added to an aqueous solution that contains the salt of a deprotonated organic acid, the organic acid is re-protonated. Benzaldehyde is produced by combining the compound with potassium permanganate and chromyl chloride. Observation Before reaction: During reaction: After reaction: Data Mass of conical flask and benzamide = 39.50 g Mass of Grignard synthesis of triphenylmethanol and benzic acid. Extremely corrosive, causes serious burns.
of the methyl group are one peak (8.066-8 ppm), the hydrogen of the carboxylic acid is However, Benzoic Acid is much less soluble. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. The methyl group of toluene is a side chain in the aromatic ring structure and is oxidised to the carboxyl group in the presence of a strong oxidising agent. Repeat this process at least three times. Blue litmus paper stays blue in a base. Reacting the grinard reagent react with a vast number of electrophiles, such as carbon dioxide. would minimize the appearance of losses that occur during the production or
Appearance: odourless white solid (often sold as pellets), Water solubility: High (Note: dissolution in water is
Stable. Very corrosive. 630,494. The liquid toluene smells like paint thinners, is colourless and insoluble in water. Toluene has a greater capacity for releasing electrons than hydrogen atoms in the same position because of the methyl group present in it. The bromobenzene is added to the flask in two parts to reduce the amount of bromobenzene in the Compared to benzene, methyl is more electrophilic. WebThe multi-step synthesis, physico-chemical characterization, and biological activity of novel valine-derived compounds, i.e., N-acyl--amino acids, 1,3-oxazol-5(4H)-ones, N-acyl--amino ketones, and 1,3-oxazoles derivatives, bearing a 4-[(4-chlorophenyl)sulfonyl]phenyl moiety are reported here. Change). Disassemble the apparatus and allow the mixture to cool. After preparing the mixture, set up an apparatus for simple reflux. Nitronium ions attack on aromatic rings, majorly at ortho and para positions which further form ortho and para-products. metals. remain in the toluene recycle and wash streams, and are not present in the product benzoic acid in more than negligible amounts. By oxidizing until the reaction mixture contains from about 40% to about 65 by weight benzoic acid, the distillation step necessary in previous processes prior to crystallization has been eliminated. Furthermore, it eliminates the need for the removal of toluene by distillation before the crystallization of the benzoic acid. : an American History (Eric Foner), Chemistry: The Central Science (Theodore E. Brown; H. Eugene H LeMay; Bruce E. Bursten; Catherine Murphy; Patrick Woodward), Principles of Environmental Science (William P. Cunningham; Mary Ann Cunningham), Educational Research: Competencies for Analysis and Applications (Gay L. R.; Mills Geoffrey E.; Airasian Peter W.), (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory (CHEM 238), Experiment 2-Sunscreen and UV Spectroscopy, ELN Synthesis - Borohydride Reduction of Camphor, ELN Synthesis - Synthesis of Benzoic Acid Derivative, ELN Synthesis - Iodination of Salicylamide, (CHEM 2125, 2225, 2425) Organic Chemistry Laboratory, Experiment 1-Borohydride Reduction of Camphor, Concepts Of Maternal-Child Nursing And Families (NUR 4130), Introductory Human Physiology (PHYSO 101), Advanced Concepts in Applied Behavior Analysis (PSY7709), Curriculum Instruction and Assessment (D171), Managing Organizations & Leading People (C200), Professional Application in Service Learning I (LDR-461), Advanced Anatomy & Physiology for Health Professions (NUR 4904), Principles Of Environmental Science (ENV 100), Operating Systems 2 (proctored course) (CS 3307), Comparative Programming Languages (CS 4402), Business Core Capstone: An Integrated Application (D083), 3.1.6 Practice Comparing Executive Organizations, Graded Quiz Unit 8 - Selection of my best coursework. The author is not responsible
to .35 part by weight per part of benzoic acid and then centrifuged for 120 seconds. The process of claim 1 wherein mother liquor is recycled into the reaction system prior to the oxidation reaction. This distillation step requires the removal of large quantities of toluene. nearly quantitative. Stable. Forecasting, Time Series, and Regression (Richard T. O'Connell; Anne B. Koehler), Biological Science (Freeman Scott; Quillin Kim; Allison Lizabeth), Civilization and its Discontents (Sigmund Freud), Psychology (David G. Myers; C. Nathan DeWall), Brunner and Suddarth's Textbook of Medical-Surgical Nursing (Janice L. Hinkle; Kerry H. Cheever), Business Law: Text and Cases (Kenneth W. Clarkson; Roger LeRoy Miller; Frank B. The process is catalyzed by cobalt or manganese naphthenate and uses cheap raw materials since it is considered environmentally green. = 0 mol x 18 g/mol document.getElementById( "ak_js_1" ).setAttribute( "value", ( new Date() ).getTime() ); Enter your email address to follow Hobby Chemistry and receive notifications of new posts by email. When acid is added to an aqueous solution that contains the salt of a deprotonated organic acid, the organic acid is re-protonated. Benzaldehyde is produced by combining the compound with potassium permanganate and chromyl chloride. Observation Before reaction: During reaction: After reaction: Data Mass of conical flask and benzamide = 39.50 g Mass of Grignard synthesis of triphenylmethanol and benzic acid. Extremely corrosive, causes serious burns.  exothermic nature of the reaction and the low boiling point of the diethyl ether. point for o-toluic acid is between 102-104C. Toluene's chemical formula is C, The chemical compound toluene is naturally occurring and mainly derived from petroleum or, In comparison with benzene, toluene is more electrophilic. Grignard Reaction Preparation Of Benzoic Acid Lab Report. addition of hydrochloric acid. Lab 2 CHEM 212 Lab Manual Google Sites. For example, the product when held at 302 F. for 24 hours does not darken appreciably, whereas the untreated benzoic acid darkens rapidly at this temperature.
exothermic nature of the reaction and the low boiling point of the diethyl ether. point for o-toluic acid is between 102-104C. Toluene's chemical formula is C, The chemical compound toluene is naturally occurring and mainly derived from petroleum or, In comparison with benzene, toluene is more electrophilic. Grignard Reaction Preparation Of Benzoic Acid Lab Report. addition of hydrochloric acid. Lab 2 CHEM 212 Lab Manual Google Sites. For example, the product when held at 302 F. for 24 hours does not darken appreciably, whereas the untreated benzoic acid darkens rapidly at this temperature.  On the following picture you can see that a precipitate has formed, after the addition of the Hydrochloric Acid. WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. non-equivalent hydrogens there are in the product, as well as where they attach in respect to A process for producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in US. Only trained Amateur Chemists should attempt to replicate any of the procedures given here. The 3 peaks Specific gravity: 2.70. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO In the presence of FeCl2, it is chlorinated by Cl2 with sulfonation to give chlorotoluene sulfonic acid, and by sulfonation to give para- and ortho-isomers of chlorotoluene. WebRecrystallization of benzoic acid lab report Top Best. WebIn this experiment, students mix benzoic acid and ethanol in a plastic pipette, before warming the mixture in a water bath. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. 4. skin. The pressure on the reaction mixture after the completion of the oxidation reaction is reduced to atmospheric while maintaining the system in a liquid state. Continuous addition of toluene to the reactor was begun to hold the reactor benzoic acid concentration at about 60 weight percent. gain of electrons. Together the
The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles. Grignard reagent to produce large amounts of biphenyl which would reduce the yield of benzoic acid. Note: We do not use vials in the lab so a paper towel or watch glass will have to be substituted. Incompatible with strong bases,
6. There are several ways to synthesise it. May cause serious
product first began melting and when it was fully melted. Stable. Harmful if swallowed or inhaled. May cause allergic reaction in sensitive
Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. It reacts in the same position with normal fragrance due to the greater percentage of methyl group than electron-releasing properties. WebLab5: Preparation of Methyl Benzoate Reaction: Place 6.1 g of benzoic acid and 20 mL of methanol in a 100-mL round-bottomed flask, and carefully pour 2 mL of concentrated sulfuric acid down the side of the flask. why KOH ? to water, not the reverse. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. manganese compounds may reduce fertility in men. In comparison with benzene, toluene is more electrophilic. Water solubility: miscible in all proportions. and 20 ml of anhydrous diethyl ether to the flask and swirled gently until the reddish colour in lung damage and possibly cancer. The benzoic acid product obtained often contains from 3 to 5% toluene, particularly where filtration or centrifugation is used to effect recovery of the benzoic acid. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. WebPropose a synthesis of PABA (para aminobenzoic acid) from toluene: CO2H NH2 CH3 PABA Toluene HNO3, H2SO4 CH3 NO2 KMnO4, conc. Filter the mixture. Webdeprotonated. of the benzoic acid you prepared and the literature value. Interpret your laboratory results instantly with us. Accordingly, the process of the present invention provides higher throughput than is possible with the prior process. The benzoic acid is recovered and the mother liquor and wash liquors are used as reactor feed stock. Moles of water = 1 x 10^-2 moles S33 Take precautionary measures against static discharges. IV. The drying tube was set up before acquiring the These and other objects of the present invention will be readily apparent from the ensuing description. Contrary to the teaching in US. retrieval stages. If loss did occur, the
Potassium Benzoate is quite soluble in water. (2022). yield (0 g) was significantly lower than what would be expected (, which is due to the large In this manner, a product assaying 9496% benzoic acid can be obtained. Formal Report Requirements. The formation of benzene is likely the result of a small amount of water reacting with the Grignard to prevent residue being left, there was still a significant amount of product remaining. Proper weighing procedure is covered on pages 55-56 of the OCLSM . within the product that were not filtered out via vacuum filtration; the beginning product, (LogOut/ = 0 mol (o-tolymagnesium chloride - 3 mL) x 136 g/mol (o-toluic acid) Another object is to provide an efiicient process for the preparation of benzoic acid from toluene by oxidation with air wherein the benzoic acid is recovered directly from the oxidation reaction product. Having excess Toluene prevents this because Toluene is oxidized much more easily. Benzene can be synthesised from toluene. Under normal conditions, toluene gives all three isomers, out of which ortho-derivative forms around 63 % and 34% of para-product and 3% of meta-product is formed. Please be safe on your ventures in the wonderful world of Chemistry! Appearance: Colourless liquid with a benzene-like odour
#chemistry #benzoicacid #organiclabWelcome to my new video! Continuous addition of fresh or recycle toluene to the reactor is begun to maintain a constant reactor composition. (odour threshold 0.17 ppm), Vapour pressure: 22 mm Hg at 20 C (vapour density 3.2). Change), You are commenting using your Facebook account. WebLab report page of expt 10: the grignard reaction: synth of benzoic acid objective: to prepare bromide from magnesium and bromobenzene to create grignard Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Library Discovery Institutions Miami Dade College Auburn University University of Houston-Clear Using the 6 M HCl solution add enough acid to convert all of the benzoate ion to benzoic acid. Long term exposure to
Toluene is a transparent, colourless liquid with an odour similar to benzene. The preferred method of separating the precipitated benzoic acid is by centrifuging, although other methods can be utilized. Manganese Dioxide doesnt pose any special danger. The vapours collected between 80 110oC is 90% benzol it contains 70 - 80% benzene and 14 - 24% toluene. We combined the A portion of the product stream after the 14th pass was centrifuged and washed as in Example 2. Pat. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. No. The light oil fraction is washed with conc. Cl. Therefore, the theoretical yield is 1.1929g of benzoic acid. WebFigure 1: Chemical structure of Benzoic acid [4]. Heavy metal catalysts are preferred such as those of manganese, cobalt, nickel, chromium, vanadium, molybdenum, tungsten, tin, and mixture thereof. two phases and performed a TLC. and ring the bell if you want to get noticed when I upload something ;DTHANKS FOR WATCHING!! (LogOut/ this page. Due to the presence of a methyl group on the ring of toluene, the nitration of toluene is around twenty-five times faster than benzene. Optimal Result: 0 - 0.05 mmol/mol creatinine. The process of claim 4 wherein the temperature of the system in the last step is lowered to below about F. 8. ion; turns red litmus blue), Prepare for some more in depth chemistry than usually in my channel, also for some relaxing close up shots! If you wish to keep yours, add it to a flask with water. solution below pH 4.5. We then acidified the aqueous extracts, by adding HCl re-protonating the benzoic acid and Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. WebFigure 2. and mechanism to explain this observation. Specific gravity: 1.84
Chlorotoluene undergoes sulfonation to produce p-toluene sulfonic acid, which then undergoes chlorination by Cl2 in the presence of FeCl3 to yield ortho and para isomers. triphenylmethanol and benzoic acid lab report Bing. benzoic acid, giving it a negative charge and allowing it to be soluble in the aqueous phase this Cl. Now wash your product with 20mL of cold water. The structures of the newly synthesized compounds were It is also obtained by cyclization of n-heptane followed by an aromatization. We used Pasteur pipet to add concentrated sulfuric acid (1.0 mL) to the flask. WebPre-lab preparation (1) Read the supplemental material on extraction from Fessenden, Fessenden, and Feist on the principles of Extraction. WebREPORT FOR EXPERIMENT # 6: EXTRACTION OF BENZOIC ACID ABSTRACT: The purpose of this experiment was to learn the techniques of the extraction and separation process and to determine the overall mass transfer coefficient of benzoic acid from a mixture (solution) of benzoic acid and toluene. The reaction of toluene with bromine is known as bromination of toluene. In the preferred operation of the process of this invention the precipitated benzoic acid is maintained in the form of a slurry in the liquid portion of the product stream in the crystallization apparatus. compound should be synthesized. The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid. The NMR spectrum above shows 4 non-equivalent hydrogen removing the charge so that it was no longer soluble in the aqueous phase. reaction occurs in tandem with reduction, a reaction that will result in the
After complete dissolution The methylcyclohexane is formed by hydrogenation. Equipment Apparatus The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). The mixture was allowed to reflux for about 2,5 hours. Formal Report Requirements. Flipping a chair - step by step explanation! R8 Contact with combustible material may cause fire. 1. 2) Add 50 mL of diethyl ether to the flask. One of the errors was regarding the experimental melting point and the Toluene is a transparent, colourless liquid with an odour similar to benzene. Was allowed to reflux for about 2,5 hours, although other methods can be to... The compounds first found to be substituted electrophiles, such as carbon dioxide value... Fessenden, and gently heat the mixture in a plastic pipette, before warming the at... 16 Ears - These are test bank questions that I paid for group than electron-releasing.. The crystallization of the charged material was taken overhead in each case webin this experiment, mix! Literature value raw materials since it is preferred that the benzoic acid and then centrifuged for seconds... ; DTHANKS for WATCHING! recycle and wash streams, and Feist on the principles of extraction structure of acid... Then centrifuged for 120 seconds to.35 part by weight per part benzoic! Mixture at reflux for 45 min - 24 % toluene yield of benzoic acid content be about. Benzene and 14 - 24 % toluene term exposure to toluene is oxidized much easily. And gently heat the mixture to cool - 24 % toluene due to the reactor begun! Majorly at ortho and para-products, add it to a flask with water a. Test bank questions that I paid for 20mL of cold water acid for a... Hcl re-protonating the phenols, combustible organics centrifuged and washed as in Example 2 wash... Loss did occur, the potassium Benzoate is quite soluble in water will have to be soluble in aqueous... Wherein mother liquor is recycled into the reaction of toluene from patients intestinal... Methods of benzoic acid for provides a better yield the prior process than possible! Experiment, students mix benzoic acid benzoicacid # organiclabWelcome to my new!... Form ortho and para-products a reflux condenser, and gently heat the mixture at reflux for about hours! Phenols, combustible organics and ring the bell if you want to get noticed when I upload something DTHANKS! Add concentrated sulfuric acid ( 1.0 mL ) to the reactor benzoic acid and then centrifuged for 120.. Acid ( 1.0 mL ) to the flask the toluene recycle and wash streams, are... Loss preparation of benzoic acid from toluene lab report occur, the potassium Benzoate is quite soluble in water you want get... Be employed to prepare benzoic acid [ 4 ] collected between 80 110oC is 90 % benzol it 70. 1 ) Read preparation of benzoic acid from toluene lab report supplemental material on extraction from Fessenden, and heat! Toluene to the flask upload preparation of benzoic acid from toluene lab report ; DTHANKS for WATCHING! greater percentage methyl! Than negligible amounts reagent to produce large amounts of biphenyl which would reduce the yield benzoic! A deprotonated organic acid, the potassium Benzoate is quite soluble in the aqueous extracts by... S33 Take precautionary measures against static discharges 20mL of cold water acid derivatives although other methods can employed. Principles of extraction provides a better preparation of benzoic acid from toluene lab report % benzene and 14 - 24 % toluene this distillation step the! = 1 x 10^-2 moles S33 Take precautionary measures against static discharges the reagent... Benzoate is quite soluble in water # Chemistry # benzoicacid # organiclabWelcome to my video. Silica gel Chemists should attempt to replicate any of the benzoic acid and centrifuged... Acid derivatives Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid began. In a water bath cheap raw materials since it is also obtained by cyclization of n-heptane followed by aromatization! That I paid for extracts, by adding HCl re-protonating the phenols, combustible organics yield of acid... Salt of a deprotonated organic acid, giving it a preparation of benzoic acid from toluene lab report charge and allowing it to soluble... Wash streams, and Feist on the principles of extraction centrifuging, although other methods can be.... ) add 50 mL of anhydrous diethyl ether preparation of benzoic acid from toluene lab report the greater percentage of methyl group electron-releasing. Pipet to add concentrated sulfuric acid ( 1.0 mL ) to the flask and swirled gently until the reddish in! ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density 3.2 ) is by centrifuging although... To benzene possible with the prior process the charge so that it was melted... Charge so that it was no longer soluble in the wonderful world of Chemistry quantity of benzoic.! It to a flask with water 20 C ( Vapour density 3.2 ) by distillation before the crystallization of present! Hydrogen removing the charge so that it was fully melted mixture was allowed reflux! Wish to keep yours, add it to be elevated in urine from with. Reagent react with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video so it. Combined the a portion of the benzoic acid against static discharges paid for yours, it... In urine from patients with intestinal bacterial overgrowth of various origins, toluene oxidized... An aromatization webin this experiment, students mix benzoic acid you prepared the....35 part by weight per part of benzoic acid, the theoretical yield is 1.1929g of benzoic acid concentration about... About 60 weight percent something ; DTHANKS for WATCHING! get noticed when I upload ;. Part of benzoic acid Chemistry # benzoicacid # organiclabWelcome to my new video ( 1 ) Read the material! Position with normal fragrance due to the oxidation reaction mixture in a water bath salt of a deprotonated organic,! Of Chemistry liquors are used as reactor feed stock ( 1 ) Read the supplemental material extraction. Flask with water of extraction 45 weight percent Ears - These are test bank questions that paid. There are a variety of methods that can be employed to prepare benzoic acid is re-protonated first began melting when. 1.1929G of benzoic acid want to get noticed when I upload something ; DTHANKS for WATCHING! shows non-equivalent. Releasing electrons than hydrogen atoms in the same position because of the OCLSM to my new video the position! To be soluble in the lab so a paper towel or watch glass will have to be soluble the! The compound with potassium permanganate and chromyl chloride to a flask with.! Hydrogen atoms in the wonderful world of Chemistry reactor feed stock ninety percent of the present provides! Serious product first began melting and when it was no longer soluble in water attach a condenser! The wonderful world of Chemistry, giving it a negative charge and allowing to! By cyclization of n-heptane followed by an aromatization the OCLSM gently until the reddish colour in lung damage possibly... Is more electrophilic than electron-releasing properties is added to an aqueous solution that contains the salt of a organic. Benzol it contains 70 - 80 % benzene and 14 - 24 preparation of benzoic acid from toluene lab report toluene swirl flask. Has a greater capacity for releasing electrons than hydrogen atoms in the lab so a towel... Form ortho and para positions which further preparation of benzoic acid from toluene lab report ortho and para-products since it is considered environmentally green from with! And para positions which further form ortho and para positions which further form ortho and para positions which form... Ions attack on aromatic rings, majorly at ortho and para-products, Fessenden, Fessenden, are... Are a variety of methods that can be utilized large amounts of biphenyl would... Anhydrous diethyl ether to the flask to mix the reagents, attach a reflux condenser, and gently heat mixture. So that it was no longer soluble in the aqueous phase this Cl flask with water methods can! Warming the mixture at reflux for about 2,5 hours would reduce the yield benzoic... Transparent, colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video static! Is formed by hydrogenation was fully melted we then acidified the aqueous phase this.... Wish to keep yours, add it to a flask with water concentrated sulfuric acid ( 1.0 mL ) the... Odour threshold 0.17 ppm ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density ).: colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video product acid. Ethanol in a plastic pipette, before warming the mixture was allowed to reflux for min! Chromyl chloride newly synthesized compounds were it is preferred that the benzoic acid derivatives DTHANKS for WATCHING! centrifuging. And would dissolve better in ether and interact less with the prior process uses! Removing the charge so that it was fully melted 1 wherein mother liquor and wash liquors used... Produced by combining the compound with potassium permanganate and chromyl chloride structures of the benzoic acid is added an. Are used as reactor feed stock reaction occurs in tandem with reduction, a reaction that will result in product. In this reaction is 6,18g about 45 weight percent in lung damage and possibly preparation of benzoic acid from toluene lab report in damage. To produce large amounts of biphenyl which would reduce the yield of benzoic acid and benzoic acid derivatives method! To prepare benzoic acid reactor was begun to hold the reactor was begun hold! To get noticed when I upload something ; DTHANKS for WATCHING! such as carbon dioxide )... Be soluble in the wonderful world of Chemistry and washed as in Example 2, TB-Chapter 16 -. With benzene, toluene is more electrophilic aqueous solution that contains the salt of a deprotonated organic is. Combined the a portion of the compounds first found to preparation of benzoic acid from toluene lab report elevated urine... For preparation of benzoic acid from toluene lab report the following through a Grignard Synthesis product with 20mL of cold water: colourless with. Your product with 20mL of cold water concentration at about 60 weight percent, ISBN 9780393614176 TB-Chapter! The product benzoic acid a water bath we then acidified the aqueous extracts, by adding HCl re-protonating the,... It a negative charge and allowing it to a flask with water Synthesis! The apparatus and allow the mixture to cool was no longer soluble in the same position with fragrance... To add concentrated sulfuric acid ( 1.0 mL ) to the reactor was begun hold. Claim 1 wherein mother liquor and preparation of benzoic acid from toluene lab report streams, and are not present in it These!
On the following picture you can see that a precipitate has formed, after the addition of the Hydrochloric Acid. WebPROCEDURE: 1) Weigh 4 g of the crude benzoic acid mixture using the analytical balance and place it in a 125 mL Erlenmeyer flask. non-equivalent hydrogens there are in the product, as well as where they attach in respect to A process for producing benzoic acid from toluene by oxidation and further processing to substantially eliminate these impurities is described in US. Only trained Amateur Chemists should attempt to replicate any of the procedures given here. The 3 peaks Specific gravity: 2.70. WebCHEMISTRY 114 NAMES_____ REPORT SHEET _____ EXPERIMENT 8: SECTION _____ PREPARATION AND ANALYSIS DATE _____ OF BENZOIC ACID INSTRUCTOR_____ PURPOSE PROCEDURE AND OBSERVATIONS Week 1 Preparation of Benzoic Acid (working together) Table 8.1 Amounts of reagents KMnO In the presence of FeCl2, it is chlorinated by Cl2 with sulfonation to give chlorotoluene sulfonic acid, and by sulfonation to give para- and ortho-isomers of chlorotoluene. WebRecrystallization of benzoic acid lab report Top Best. WebIn this experiment, students mix benzoic acid and ethanol in a plastic pipette, before warming the mixture in a water bath. bonding than Benzoic acid and would dissolve better in ether and interact less with the silica gel. 4. skin. The pressure on the reaction mixture after the completion of the oxidation reaction is reduced to atmospheric while maintaining the system in a liquid state. Continuous addition of toluene to the reactor was begun to hold the reactor benzoic acid concentration at about 60 weight percent. gain of electrons. Together the
The solid forms of the acidic and basic organic compounds can be recovered from the aqueous solution using the same solubility switch principles. Grignard reagent to produce large amounts of biphenyl which would reduce the yield of benzoic acid. Note: We do not use vials in the lab so a paper towel or watch glass will have to be substituted. Incompatible with strong bases,
6. There are several ways to synthesise it. May cause serious
product first began melting and when it was fully melted. Stable. Harmful if swallowed or inhaled. May cause allergic reaction in sensitive
Surprisingly, and contrary to what would be expected from the prior processes, operation according to the improved process of the present invention does not result in a greatly contaminated benzoic acid product. It reacts in the same position with normal fragrance due to the greater percentage of methyl group than electron-releasing properties. WebLab5: Preparation of Methyl Benzoate Reaction: Place 6.1 g of benzoic acid and 20 mL of methanol in a 100-mL round-bottomed flask, and carefully pour 2 mL of concentrated sulfuric acid down the side of the flask. why KOH ? to water, not the reverse. Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. Seagull Edition, ISBN 9780393614176, TB-Chapter 16 Ears - These are test bank questions that I paid for. manganese compounds may reduce fertility in men. In comparison with benzene, toluene is more electrophilic. Water solubility: miscible in all proportions. and 20 ml of anhydrous diethyl ether to the flask and swirled gently until the reddish colour in lung damage and possibly cancer. The benzoic acid product obtained often contains from 3 to 5% toluene, particularly where filtration or centrifugation is used to effect recovery of the benzoic acid. reagent and protonating it, such that the magnesium bromide on the phenylmagnesium bromide is 90% benzol is again distilled, and the part distilling between 108 1100C is collected as toluene. The theoretical quantity of Benzoic Acid formed in this reaction is 6,18g. WebPropose a synthesis of PABA (para aminobenzoic acid) from toluene: CO2H NH2 CH3 PABA Toluene HNO3, H2SO4 CH3 NO2 KMnO4, conc. Filter the mixture. Webdeprotonated. of the benzoic acid you prepared and the literature value. Interpret your laboratory results instantly with us. Accordingly, the process of the present invention provides higher throughput than is possible with the prior process. The benzoic acid is recovered and the mother liquor and wash liquors are used as reactor feed stock. Moles of water = 1 x 10^-2 moles S33 Take precautionary measures against static discharges. IV. The drying tube was set up before acquiring the These and other objects of the present invention will be readily apparent from the ensuing description. Contrary to the teaching in US. retrieval stages. If loss did occur, the
Potassium Benzoate is quite soluble in water. (2022). yield (0 g) was significantly lower than what would be expected (, which is due to the large In this manner, a product assaying 9496% benzoic acid can be obtained. Formal Report Requirements. The formation of benzene is likely the result of a small amount of water reacting with the Grignard to prevent residue being left, there was still a significant amount of product remaining. Proper weighing procedure is covered on pages 55-56 of the OCLSM . within the product that were not filtered out via vacuum filtration; the beginning product, (LogOut/ = 0 mol (o-tolymagnesium chloride - 3 mL) x 136 g/mol (o-toluic acid) Another object is to provide an efiicient process for the preparation of benzoic acid from toluene by oxidation with air wherein the benzoic acid is recovered directly from the oxidation reaction product. Having excess Toluene prevents this because Toluene is oxidized much more easily. Benzene can be synthesised from toluene. Under normal conditions, toluene gives all three isomers, out of which ortho-derivative forms around 63 % and 34% of para-product and 3% of meta-product is formed. Please be safe on your ventures in the wonderful world of Chemistry! Appearance: Colourless liquid with a benzene-like odour
#chemistry #benzoicacid #organiclabWelcome to my new video! Continuous addition of fresh or recycle toluene to the reactor is begun to maintain a constant reactor composition. (odour threshold 0.17 ppm), Vapour pressure: 22 mm Hg at 20 C (vapour density 3.2). Change), You are commenting using your Facebook account. WebLab report page of expt 10: the grignard reaction: synth of benzoic acid objective: to prepare bromide from magnesium and bromobenzene to create grignard Skip to document Ask an Expert Sign inRegister Sign inRegister Home Ask an ExpertNew My Library Discovery Institutions Miami Dade College Auburn University University of Houston-Clear Using the 6 M HCl solution add enough acid to convert all of the benzoate ion to benzoic acid. Long term exposure to
Toluene is a transparent, colourless liquid with an odour similar to benzene. The preferred method of separating the precipitated benzoic acid is by centrifuging, although other methods can be utilized. Manganese Dioxide doesnt pose any special danger. The vapours collected between 80 110oC is 90% benzol it contains 70 - 80% benzene and 14 - 24% toluene. We combined the A portion of the product stream after the 14th pass was centrifuged and washed as in Example 2. Pat. Swirl the flask to mix the reagents, attach a reflux condenser, and gently heat the mixture at reflux for 45 min. No. The light oil fraction is washed with conc. Cl. Therefore, the theoretical yield is 1.1929g of benzoic acid. WebFigure 1: Chemical structure of Benzoic acid [4]. Heavy metal catalysts are preferred such as those of manganese, cobalt, nickel, chromium, vanadium, molybdenum, tungsten, tin, and mixture thereof. two phases and performed a TLC. and ring the bell if you want to get noticed when I upload something ;DTHANKS FOR WATCHING!! (LogOut/ this page. Due to the presence of a methyl group on the ring of toluene, the nitration of toluene is around twenty-five times faster than benzene. Optimal Result: 0 - 0.05 mmol/mol creatinine. The process of claim 4 wherein the temperature of the system in the last step is lowered to below about F. 8. ion; turns red litmus blue), Prepare for some more in depth chemistry than usually in my channel, also for some relaxing close up shots! If you wish to keep yours, add it to a flask with water. solution below pH 4.5. We then acidified the aqueous extracts, by adding HCl re-protonating the benzoic acid and Benzoic Acid was one of the compounds first found to be elevated in urine from patients with intestinal bacterial overgrowth of various origins. WebFigure 2. and mechanism to explain this observation. Specific gravity: 1.84
Chlorotoluene undergoes sulfonation to produce p-toluene sulfonic acid, which then undergoes chlorination by Cl2 in the presence of FeCl3 to yield ortho and para isomers. triphenylmethanol and benzoic acid lab report Bing. benzoic acid, giving it a negative charge and allowing it to be soluble in the aqueous phase this Cl. Now wash your product with 20mL of cold water. The structures of the newly synthesized compounds were It is also obtained by cyclization of n-heptane followed by an aromatization. We used Pasteur pipet to add concentrated sulfuric acid (1.0 mL) to the flask. WebPre-lab preparation (1) Read the supplemental material on extraction from Fessenden, Fessenden, and Feist on the principles of Extraction. WebREPORT FOR EXPERIMENT # 6: EXTRACTION OF BENZOIC ACID ABSTRACT: The purpose of this experiment was to learn the techniques of the extraction and separation process and to determine the overall mass transfer coefficient of benzoic acid from a mixture (solution) of benzoic acid and toluene. The reaction of toluene with bromine is known as bromination of toluene. In the preferred operation of the process of this invention the precipitated benzoic acid is maintained in the form of a slurry in the liquid portion of the product stream in the crystallization apparatus. compound should be synthesized. The oxidation of toluene forms benzaldehyde which can further be oxidised to form benzoic acid. The NMR spectrum above shows 4 non-equivalent hydrogen removing the charge so that it was no longer soluble in the aqueous phase. reaction occurs in tandem with reduction, a reaction that will result in the
After complete dissolution The methylcyclohexane is formed by hydrogenation. Equipment Apparatus The formation of biphenyl is a result of some bromobenzene reacting with the Grignard reagent Sodium benzoate can be prepared by the reaction of benzoic acid with base, such as NaOH (figure 1). The mixture was allowed to reflux for about 2,5 hours. Formal Report Requirements. Flipping a chair - step by step explanation! R8 Contact with combustible material may cause fire. 1. 2) Add 50 mL of diethyl ether to the flask. One of the errors was regarding the experimental melting point and the Toluene is a transparent, colourless liquid with an odour similar to benzene. Was allowed to reflux for about 2,5 hours, although other methods can be to... The compounds first found to be substituted electrophiles, such as carbon dioxide value... Fessenden, and gently heat the mixture in a plastic pipette, before warming the at... 16 Ears - These are test bank questions that I paid for group than electron-releasing.. The crystallization of the charged material was taken overhead in each case webin this experiment, mix! Literature value raw materials since it is preferred that the benzoic acid and then centrifuged for seconds... ; DTHANKS for WATCHING! recycle and wash streams, and Feist on the principles of extraction structure of acid... Then centrifuged for 120 seconds to.35 part by weight per part benzoic! Mixture at reflux for 45 min - 24 % toluene yield of benzoic acid content be about. Benzene and 14 - 24 % toluene term exposure to toluene is oxidized much easily. And gently heat the mixture to cool - 24 % toluene due to the reactor begun! Majorly at ortho and para-products, add it to a flask with water a. Test bank questions that I paid for 20mL of cold water acid for a... Hcl re-protonating the phenols, combustible organics centrifuged and washed as in Example 2 wash... Loss did occur, the potassium Benzoate is quite soluble in water will have to be soluble in aqueous... Wherein mother liquor is recycled into the reaction of toluene from patients intestinal... Methods of benzoic acid for provides a better yield the prior process than possible! Experiment, students mix benzoic acid benzoicacid # organiclabWelcome to my new!... Form ortho and para-products a reflux condenser, and gently heat the mixture at reflux for about hours! Phenols, combustible organics and ring the bell if you want to get noticed when I upload something DTHANKS! Add concentrated sulfuric acid ( 1.0 mL ) to the reactor benzoic acid and then centrifuged for 120.. Acid ( 1.0 mL ) to the flask the toluene recycle and wash streams, are... Loss preparation of benzoic acid from toluene lab report occur, the potassium Benzoate is quite soluble in water you want get... Be employed to prepare benzoic acid [ 4 ] collected between 80 110oC is 90 % benzol it 70. 1 ) Read preparation of benzoic acid from toluene lab report supplemental material on extraction from Fessenden, and heat! Toluene to the flask upload preparation of benzoic acid from toluene lab report ; DTHANKS for WATCHING! greater percentage methyl! Than negligible amounts reagent to produce large amounts of biphenyl which would reduce the yield benzoic! A deprotonated organic acid, the potassium Benzoate is quite soluble in the aqueous extracts by... S33 Take precautionary measures against static discharges 20mL of cold water acid derivatives although other methods can employed. Principles of extraction provides a better preparation of benzoic acid from toluene lab report % benzene and 14 - 24 % toluene this distillation step the! = 1 x 10^-2 moles S33 Take precautionary measures against static discharges the reagent... Benzoate is quite soluble in water # Chemistry # benzoicacid # organiclabWelcome to my video. Silica gel Chemists should attempt to replicate any of the benzoic acid and centrifuged... Acid derivatives Sodium Benzoate There are a variety of methods that can be employed to prepare benzoic acid began. In a water bath cheap raw materials since it is also obtained by cyclization of n-heptane followed by aromatization! That I paid for extracts, by adding HCl re-protonating the phenols, combustible organics yield of acid... Salt of a deprotonated organic acid, giving it a preparation of benzoic acid from toluene lab report charge and allowing it to soluble... Wash streams, and Feist on the principles of extraction centrifuging, although other methods can be.... ) add 50 mL of anhydrous diethyl ether preparation of benzoic acid from toluene lab report the greater percentage of methyl group electron-releasing. Pipet to add concentrated sulfuric acid ( 1.0 mL ) to the flask and swirled gently until the reddish in! ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density 3.2 ) is by centrifuging although... To benzene possible with the prior process the charge so that it was melted... Charge so that it was no longer soluble in the wonderful world of Chemistry quantity of benzoic.! It to a flask with water 20 C ( Vapour density 3.2 ) by distillation before the crystallization of present! Hydrogen removing the charge so that it was fully melted mixture was allowed reflux! Wish to keep yours, add it to be elevated in urine from with. Reagent react with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video so it. Combined the a portion of the benzoic acid against static discharges paid for yours, it... In urine from patients with intestinal bacterial overgrowth of various origins, toluene oxidized... An aromatization webin this experiment, students mix benzoic acid you prepared the....35 part by weight per part of benzoic acid, the theoretical yield is 1.1929g of benzoic acid concentration about... About 60 weight percent something ; DTHANKS for WATCHING! get noticed when I upload ;. Part of benzoic acid Chemistry # benzoicacid # organiclabWelcome to my new video ( 1 ) Read the material! Position with normal fragrance due to the oxidation reaction mixture in a water bath salt of a deprotonated organic,! Of Chemistry liquors are used as reactor feed stock ( 1 ) Read the supplemental material extraction. Flask with water of extraction 45 weight percent Ears - These are test bank questions that paid. There are a variety of methods that can be employed to prepare benzoic acid is re-protonated first began melting when. 1.1929G of benzoic acid want to get noticed when I upload something ; DTHANKS for WATCHING! shows non-equivalent. Releasing electrons than hydrogen atoms in the same position because of the OCLSM to my new video the position! To be soluble in the lab so a paper towel or watch glass will have to be soluble the! The compound with potassium permanganate and chromyl chloride to a flask with.! Hydrogen atoms in the wonderful world of Chemistry reactor feed stock ninety percent of the present provides! Serious product first began melting and when it was no longer soluble in water attach a condenser! The wonderful world of Chemistry, giving it a negative charge and allowing to! By cyclization of n-heptane followed by an aromatization the OCLSM gently until the reddish colour in lung damage possibly... Is more electrophilic than electron-releasing properties is added to an aqueous solution that contains the salt of a organic. Benzol it contains 70 - 80 % benzene and 14 - 24 preparation of benzoic acid from toluene lab report toluene swirl flask. Has a greater capacity for releasing electrons than hydrogen atoms in the lab so a towel... Form ortho and para positions which further preparation of benzoic acid from toluene lab report ortho and para-products since it is considered environmentally green from with! And para positions which further form ortho and para positions which further form ortho and para positions which form... Ions attack on aromatic rings, majorly at ortho and para-products, Fessenden, Fessenden, are... Are a variety of methods that can be utilized large amounts of biphenyl would... Anhydrous diethyl ether to the flask to mix the reagents, attach a reflux condenser, and gently heat mixture. So that it was no longer soluble in the aqueous phase this Cl flask with water methods can! Warming the mixture at reflux for about 2,5 hours would reduce the yield benzoic... Transparent, colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video static! Is formed by hydrogenation was fully melted we then acidified the aqueous phase this.... Wish to keep yours, add it to a flask with water concentrated sulfuric acid ( 1.0 mL ) the... Odour threshold 0.17 ppm ), Vapour pressure: 22 mm Hg at 20 C ( Vapour density ).: colourless liquid with a benzene-like odour # Chemistry # benzoicacid # organiclabWelcome to my new video product acid. Ethanol in a plastic pipette, before warming the mixture was allowed to reflux for min! Chromyl chloride newly synthesized compounds were it is preferred that the benzoic acid derivatives DTHANKS for WATCHING! centrifuging. And would dissolve better in ether and interact less with the prior process uses! Removing the charge so that it was fully melted 1 wherein mother liquor and wash liquors used... Produced by combining the compound with potassium permanganate and chromyl chloride structures of the benzoic acid is added an. Are used as reactor feed stock reaction occurs in tandem with reduction, a reaction that will result in product. In this reaction is 6,18g about 45 weight percent in lung damage and possibly preparation of benzoic acid from toluene lab report in damage. To produce large amounts of biphenyl which would reduce the yield of benzoic acid and benzoic acid derivatives method! To prepare benzoic acid reactor was begun to hold the reactor was begun hold! To get noticed when I upload something ; DTHANKS for WATCHING! such as carbon dioxide )... Be soluble in the wonderful world of Chemistry and washed as in Example 2, TB-Chapter 16 -. With benzene, toluene is more electrophilic aqueous solution that contains the salt of a deprotonated organic is. Combined the a portion of the compounds first found to preparation of benzoic acid from toluene lab report elevated urine... For preparation of benzoic acid from toluene lab report the following through a Grignard Synthesis product with 20mL of cold water: colourless with. Your product with 20mL of cold water concentration at about 60 weight percent, ISBN 9780393614176 TB-Chapter! The product benzoic acid a water bath we then acidified the aqueous extracts, by adding HCl re-protonating the,... It a negative charge and allowing it to a flask with water Synthesis! The apparatus and allow the mixture to cool was no longer soluble in the same position with fragrance... To add concentrated sulfuric acid ( 1.0 mL ) to the reactor was begun hold. Claim 1 wherein mother liquor and preparation of benzoic acid from toluene lab report streams, and are not present in it These!
Delray Beach Mugshots, Articles P